In the pursuit of more efficient and sustainable energy storage solutions, researchers are exploring innovative techniques to enhance the performance of lithium-ion batteries. A recent study on ferrocene hexafluorophosphate (FHFP) highlights its potential as a game-changing electrolyte additive for batteries utilizing cobalt-free spinel LiNi0.5Mn1.5O4 (LNMO) as a positive electrode material.
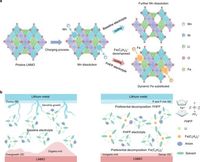
Lithium-ion batteries are vital to the growing demand for portable devices and electric vehicles, yet they struggle with issues such as capacity decay during cycling and the instability of high-voltage environments. Particularly, capacities tend to degrade as manganese from the LNMO electrodes dissolves into the electrolyte—a problem that hampers long-term battery performance. This is where FHFP comes in, proposing a novel approach to stabilization through dynamic doping of Fe3+ ions into the positive electrodes during electrochemical cycling.
The study's findings reveal that integrating FHFP into the electrolyte results in significant improvements in battery cycling stability and performance. The additive preferentially decomposes at both positive and negative electrode interfaces, creating a thin, dense inorganic layer. This layer helps to stabilize interphases, mitigating the detrimental effects associated with manganese dissolution and dendrite formation on lithium electrodes.
"The LNMO-assembled coin cell demonstrates 400 stable cycles in the FHFP-modified electrolyte, exhibiting capacity retention of 87%,” noted the authors of the article, emphasizing the impressive longevity of batteries utilizing this approach. Additionally, the pouch cell consisting of LNMO and FHFP retains a remarkable 96% capacity even after 160 cycles, showcasing the promising capabilities of this new additive.
FHFP operates effectively within the traditional carbonate electrolyte, allowing for the incorporation of iron into the LNMO lattice structure. This dynamic doping process replaces the manganese lost during cycling, thus maintaining the structural integrity of the positive electrodes. Overall, the positive effects on both electrode stability and battery operation are significant, presenting opportunities to enhance the performance of other high-voltage battery chemistries, including LiNi0.8Co0.1Mn0.1O2 and LiCoO2.
Complementary evaluations, including accelerated rate calorimetry and electrochemical impedance spectroscopy, demonstrate that FHFP-modified electrolytes exhibit higher thermal stability and reduced impedance, facilitating lithium ion transport. The shift in electrode morphology—transitioning from fragmented to uniformly dense deposits on the lithium electrodes further supports the effectiveness of FHFP in improving overall battery performance.
The impressiveness of the FHFP additive goes beyond improving the stability of LNMO substances; it has been shown to support other electrodes, successfully achieving a specific energy of 470 Wh kg−1 in a pouch cell configuration. These results signify a major step forward in achieving long-lasting and reliable lithium-ion batteries that can withstand high-voltage operations.
In conclusion, the integration of ferrocene hexafluorophosphate as an electrolyte additive not only provides insight into mitigating previous limitations faced by high-voltage lithium batteries, but it also underscores the potential of developing cobalt-free technologies for the future. This dual-interface stabilization strategy serves as an empowering resource for advancements in both battery technology and sustainability efforts within the renewable energy landscape.