In an era where nanotechnology continues to revolutionize material science, researchers have made significant strides in understanding the fibrogenic potential of metal oxide nanoparticles (MeONPs). A new study presents a comprehensive multimodal feature fusion analysis framework, which aims to predict chronic lung injuries related to exposure to MeONPs, a pressing public health concern as usage expands in various industries.
Past studies have raised alarms regarding the chronic injuries, such as fibrosis and carcinogenesis, that nanoparticles can induce, necessitating a rapid assessment of their hazards. While in silico analysis has become a common tool for the risk assessment of chemicals, predicting chronic in vivo nanotoxicity remains challenging due to the complex interactions that occur at different biological interfaces. This latest research successfully addresses these challenges by developing a predictive model based on the interactions of various MeONPs with lung tissues.
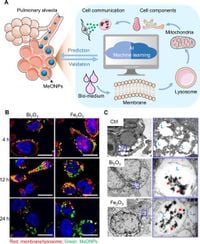
The researchers identified 87 features crucial to understanding how MeONPs interact with the lungs. These features are derived from examinations of MeONP-lung interactions and were employed in combination with machine learning techniques to reveal patterns related to chronic lung injuries, particularly fibrosis.
Conducted by a team of scientists, the study meticulously examined how particle size, surface charge, and the interaction with lysosomes affected cell damage and cytokine production in macrophages and epithelial cells. The analysis culminated in a framework that achieved a remarkable 85% accuracy rate in predicting the fibrogenic potential of various MeONPs in female mice.
The researchers emphasized that chronic exposure to nanoparticles poses substantial risks of respiratory injuries, highlighting the need for rigorous risk assessments of engineered nanomaterials. With over 10,000 nanoproducts available globally, inhalation is the primary route of exposure, which can lead to serious health complications, including lung inflammation and fibrogenesis.
The framework was developed using a library of 52 MeONPs, and the resulting dataset offers insights into how these particles could be evaluated for their fibrogenic properties without the extensive use of animal testing. The predictive model is particularly significant as it addresses the gap in reliable models for chronic respiratory toxicity, specifically targeting lung fibrosis.
To create the model, the researchers employed a variety of machine learning algorithms, identifying the random forest approach as delivering the best predictive performance. The framework stemmed from a comprehensive investigation into MeONP interactions at multiple levels—including nano-bio interfaces and responses at the cellular level--which collectively inform the model’s predictions.
“This is a new era in nanotoxicity assessment,” wrote the authors of the article, emphasizing the study’s potential benefits in regulatory decision-making and risk management of nanomaterials.
To further validate their findings, the team conducted experiments involving additional MeONPs not included in the training phase, confirming the model's efficacy in predicting fibrogenic outcomes. This robust predictive model not only advances the safety assessment landscape for nanotechnology but also introduces a mechanism-driven alternative to traditional assessments that often rely on extensive animal testing.
The development of a software tool named “Nano-induced lung fibrosis prediction” (NILFP) is an important step toward practical applications of this research. This tool aims to streamline the risk evaluation process for untested MeONPs, facilitating safer design and application of these materials in various sectors.
Understanding the intricate nature of MeONP-induced lung fibrosis—characterized by multiple biological interactions—is essential in developing early prediction models. Going forward, researchers anticipate that expanding the dataset and synthesizing new MeONPs will enhance the predictive accuracy of these models, providing a more nuanced understanding of the risks associated with engineered nanomaterials.
As engineered nanomaterials continue to proliferate in many industries, the importance of effective predictive frameworks for assessing their potential risks cannot be overstated. This research paves the way for a new approach to nanotoxicity assessment, underscoring the necessity of further investigations in the realm of nanotechnology to safeguard public health.