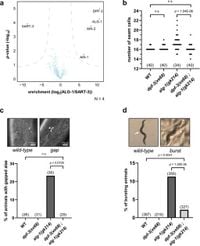
A recent study in the journal highlighted the critical function of dipeptidyl peptidase DPF-3 within the microRNA (miRNA) regulatory network in the model organism Caenorhabditis elegans. The research shows that when DPF-3 is knocked out, levels of another argonaute protein, ALG-2, increase, suggesting DPF-3's role in regulating gene expression through the miRNA mechanism.
MicroRNAs are vital to various biological processes, managing gene expression post-transcriptionally. They work by binding to messenger RNA (mRNA) and silencing its translation, either by degrading the mRNA or inhibiting its translation into proteins. In C. elegans, there are several argonautes, including ALG-1 and ALG-2, which are crucial players in this miRNA-induced silencing complex (miRISC).
The interplay between different miRNA argonautes like ALG-1 and ALG-2 and their ability to influence gene regulation has long intrigued scientists because of its implications for understanding developmental biology and gene expression regulation in more complex organisms, including humans.
In the study led by researchers, genetic experiments were conducted to determine the roles and interactions of DPF-3, ALG-1, and ALG-2 in the miRNA pathway. It was discovered that DPF-3 interacts physically and genetically with ALG-1, helping to regulate the impact of the miRNA pathway. Notably, the knockout of DPF-3 led to increased levels of ALG-2, which compensated for the loss of ALG-1, suggesting a fuller understanding of miRNA dynamics at play.
During the research process, researchers utilized mass spectrometry to identify interactors and discovered that DPF-3 is consistently found alongside known argonaute interactors. This consistency led scientists to hypothesize that DPF-3 might affect miRNA function indirectly, impacting the production and regulation of ALG-2.
The data presented showed a correlation indicating that when DPF-3 is absent, the necessary levels of ALG-2 rise, supporting the hypothesis that DPF-3 regulates the expression of this alternate argonaute. Furthermore, the research demonstrated that DPF-3 does not need to cleave ALG-2 for regulation; rather, it operates through its catalytic activities independently of direct protein interaction.
The experiments resulted in phenotypes indicative of normal miRNA activity being restored in the absence of DPF-3, thereby presenting potential pathways through which gene regulation mechanisms could adjust in response to the loss of specific components within the miRNA machinery. The findings confirm the significance of DPF-3 as a regulator by enabling proper gene expression despite compromised pathways.
Moreover, the study opens avenues for exploring similar miRNA interactions in mammals, offering a glimpse into common evolutionary mechanisms governing gene regulation across species. Understanding how miRNA pathways adapt to genetic alterations might play a crucial role in developmental biology, allowing researchers to unravel complexities in miRNA function and its downstream effects.
As the research highlights, unraveling these regulatory layers can unveil targets for future therapies and elucidate the principles underlying disorders linked with misregulation of gene expression. Such insights can spur innovation in the treatment and understanding of diseases that stem from similar biological mishaps.