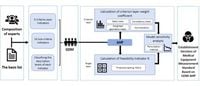
A new study outlines a structured approach to establish measurement standards for medical equipment in China's healthcare sector, leveraging a decision-making framework known as the Group Decision Making-Analytical Hierarchy Process (GDM-AHP). This innovative system aims to address the pressing need for high-quality medical services by ensuring precise calibration and measurement of medical devices, which are crucial in delivering safe and effective patient care.
The GDM-AHP evaluation system comprises five main criteria and 14 specific sub-criteria indicators, methodically organized to facilitate the feasibility assessment of implementing measurement standards across various types of medical equipment. According to the study, "the feasibility evaluation system for establishing measurement standards based on Group Decision Making-Analytical Hierarchy Processes can transform the problem of exploring the feasibility of establishing measurement standards in medical institutions into a multi-indicator quantitative evaluation problem, making the difficult-to-quantify decision-making process more scientific and objective,” wrote the authors of the article.
The necessity behind developing such a system arises from significant discrepancies in the existing measurement practices within Chinese medical institutions. Currently, the medical equipment measurement landscape faces numerous challenges: tertiary hospitals exhibit high demands for equipment and metrology services, while primary and secondary hospitals often lack timely access to measurement resources. These inconsistencies have led institutions to consider establishing internal metrology standards that could provide a stable, long-term solution for equipment measurement needs.
Historically, China's metrology practices date back to the implementation of the Metrology Law in 1985. However, as the healthcare sector continues to evolve, the existing framework has fallen short of catering to the modern demands of medical institutions. In 2014, the State Council emphasized developing third-party inspection and testing services, which highlighted the need for a more robust and responsive metrology system. Later revisions in the law, notably in 2018, established guidelines encouraging broader participation in measurement activities by qualified institutions.
The GDM-AHP method, utilized in this study, introduces a systematic approach for tackling complex decision-making problems within diverse contexts by associating qualitative assessments with quantitative outcomes. This approach breaks down the overall goal of defining measurement standards into manageable sub-goals that reflect the specific needs of different medical settings, thereby making the decision-making process more inclusive of stakeholder perspectives.
With the framework established, the authors applied the GDM-AHP evaluation system to eight different types of medical equipment across seven medical institutions with varying scales and resource allocations. Their findings demonstrated a high degree of variability in the parameters influencing the feasibility of establishing measurement standards. In particular, they noted, "establishing measurement standards is intended to ensure the quality control of medical equipment, rather than to make a profit by providing measurement services to the public,” wrote the authors of the article, highlighting the focus placed on enhancing the public health outcomes and safety over economic motivations.
As the study indicates, the initial expert judgment matrices used to determine the weights of evaluation indicators yielded a consistency score (CR) of 0.03487, indicating a satisfactory level of agreement among experts involved in the assessment process. This level of consensus was essential for ensuring the validity of the chosen metrics and enhancing the confidence in the resulting evaluation system.
Results from applying the GDM-AHP model showcased significant differences in the values of feasibility indicators across different institutions, with tertiary medical hospitals generally yielding higher feasibility scores compared to their secondary and primary counterparts. Such outcomes demonstrate the impact of institutional scale and equipment type on the requirements and processes governing the establishment of measurement standards.
Despite the optimistic findings from the study, there remain limitations and areas requiring further research. For instance, while this model provides a structured basis for decision-making, variations in measurement demands and local institutional contexts may require ongoing adjustments to evaluation criteria to maintain relevance and applicability. Furthermore, as the authors note, developing a comprehensive feasibility system for measurement standards in private medical institutions represents an uncharted territory demanding exploration.
Ultimately, this study is a vital step toward laying down a strategic foundation for improving the medical device measurement standards in China's healthcare environments. The GDM-AHP evaluation system's construction not only promises to enhance the decision-making efficiency but also aims to establish an ecosystem of high-quality healthcare delivery, ultimately benefiting patients across the spectrum of medical institutions.