In a groundbreaking study, researchers have developed a new electrochemical method to effectively degrade the antibiotic Cephalexin in wastewater using a specially synthesized Ti/TiO2-βPbO2 electrode. This innovation addresses the growing environmental concern about the presence of pharmaceutical pollutants, particularly antibiotics, in aquatic ecosystems. The Ti/TiO2-βPbO2 electrode, modified with sodium dodecyl sulfate (SDS), achieved a remarkable degradation efficiency of 97.60% for Cephalexin, demonstrating its potential for eco-friendly wastewater treatment.
Antibiotics like Cephalexin are increasingly detected in water sources, primarily due to human and agricultural uses. Once released into the environment, these substances resist degradation through conventional wastewater treatment processes, leading to ecological disturbances, such as promoting antibiotic-resistant bacteria and disrupting aquatic life. Recognizing this urgent problem, the research team focused on developing effective and sustainable methods to mitigate pharmaceutical pollutants using advanced electrochemical techniques.
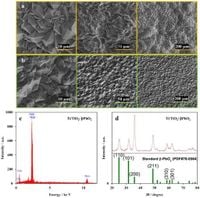
In their study, published in Scientific Reports, the team of researchers, including N. Hadavand, S. Khazalpour, L. Fotouhi, and D. Nematollahi, reported that the Ti/TiO2-βPbO2 electrode exhibited desirable electrocatalytic performance, highlighting its ability to mineralize Cephalexin effectively. The electrode was prepared using a titanium substrate coated with lead dioxide through electrochemical deposition under specific conditions, including a current density of 10 mA cm−2 for 120 minutes at 65 °C. This careful preparation aimed to enhance both the efficiency and stability of the electrode.
The efficiency of the Ti/TiO2-βPbO2 electrode was evaluated through cyclic voltammetry and differential pulse voltammetry techniques. These methods revealed that under optimal conditions, the electrode could achieve a maximum chemical oxygen demand (COD) removal efficiency of 70.27%, further validating its practical applicability in wastewater treatment.
“The synthesis process of the electrode incorporating SDS not only enhanced its electrocatalytic efficiency but also provided significant corrosion resistance, prolonging its operational lifespan,” explained the authors of the article.
The research methodology included extensive characterization of the electrode materials through various techniques, ensuring a comprehensive understanding of the degradation mechanisms involved. The study employed liquid chromatography coupled with tandem mass spectrometry (LC–MS/MS) to investigate the degradation pathways of Cephalexin, revealing insights into the breakdown products and environmental implications associated with their presence.
Preliminary results showed that the degradation increased with decreasing pollutant concentration and higher current densities. For instance, a degradation rate of 97.60% was achieved at an initial Cephalexin concentration of 50 mg L−1 under a current density of 20 mA cm−2. Even after 15 consecutive cycles, the electrode maintained a degradation efficiency of 90.18%, indicating its robustness and stability over time.
Furthermore, safety assessments of the Ti/TiO2-βPbO2 electrode revealed minimal lead ion leaching into the electrolysis solution, with concentrations measured at only 0.01 mg L−1 after prolonged use. This measurement is within the acceptable limits set by the World Health Organization for drinking water safety, emphasizing the environmentally responsible nature of this approach.
The findings of this research bear significant implications for addressing antibiotic contamination in the environment. As the research team states, “The efficacy and efficiency of the synthesized Ti/TiO2-βPbO2 electrode present a viable solution to the persistent problem of pharmaceutical pollutants in wastewater, ensuring environmental protection and promoting public health.”
This study not only advances the field of electrochemical degradation of organic pollutants but also highlights the critical need for innovative solutions in wastewater management. Researchers believe that optimizing the electrocatalytic processes and electrode materials will further enhance the degradation rates of various contaminants, paving the way for sustainable environmental practices.
As antibiotic use in healthcare and agriculture continues to rise, implementing effective treatment technologies like the Ti/TiO2-βPbO2 electrode can significantly reduce the ecological risks posed by pharmaceutical residues in water systems. The pursuit of such sustainable approaches is vital for both ecological balance and the safety of drinking water for future generations.