Leflunomide, a medication traditionally used to manage rheumatoid arthritis, has emerged in new research as a potential ally in the battle against the cardiotoxic effects of immune checkpoint inhibitors like anti-programmed death 1 (αPD1) therapy, particularly for melanoma patients. Ongoing studies indicate that this combination may alleviate heart damage without sacrificing the chemotherapy's effectiveness.
Melanoma, a devastating form of skin cancer, is responsible for approximately 5% of all skin cancer incidents and has a mortality rate of up to 65%. The introduction of αPD1 therapy has revolutionized treatment protocols, improving survival rates for about 40% of patients. However, alongside its promise, this therapy has been linked to severe side effects, notably cardiotoxicity, characterized by conditions such as myocarditis and cardiomyopathy. The urgency for interventions that can mitigate these cardiac risks without hindering anticancer efficacy has prompted a closer look at Leflunomide's capabilities.
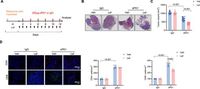
In recent experiments, researchers at Renmin Hospital of Wuhan University investigated Leflunomide's effects on αPD1-induced cardiac toxicity in melanoma-bearing mice. The study protocol involved treating one group of mice with both αPD1 and Leflunomide while a control group received αPD1 alone. Findings revealed that Leflunomide not only maintained the anticancer effects of αPD1 but also significantly improved cardiac function. This was measured through various echocardiographic parameters, including ejection fraction and cardiac output, indicating healthier heart dynamics in those receiving the combined treatment.
Significantly, the Leflunomide treatment successfully moderated the adverse cardiovascular effects associated with αPD1, which had previously resulted in detrimental changes such as decreased ejection fraction and increased cardiac injury markers. Furthermore, Leflunomide seemed to introduce beneficial modifications to the gut microbiota among the treated mice, aligning with previous studies highlighting the importance of gut health in cancer therapies.
As part of the research focus, the scientists also discovered indole-3-propionic acid (IPA)—a metabolite linked to gut bacteria—as a crucial player in mediating Leflunomide's cardioprotective effects. They determined that IPA not only acted on cardiac cells by binding to the aryl hydrocarbon receptor but also promoted beneficial signaling pathways that critically reduced cardiac inflammation and apoptosis.
This discovery underscores the significance of the microbiota-health correlation in mitigating cardiotoxic effects during cancer treatment. In practice, fecal microbiota transplantation from Leflunomide-treated mice into those solely undergoing αPD1 treatment demonstrated notable improvements in cardiac function, suggesting a direct interaction between gut microbiota composition and cardiac health during immunotherapy.
As immune checkpoint inhibitors continue to gain traction in clinical oncology, understanding how agents such as Leflunomide may help preserve cardiac health becomes ever more pressing. The prospect of retaining the life-saving potential of αPD1 while safeguarding patients from its harmful side effects would mark a significant advancement in immuno-oncology.
In conclusion, this promising evidence highlights Leflunomide as a potential therapeutic strategy in addressing αPD1-related cardiotoxicity in melanoma, primarily through influencing gut microbiota and utilizing metabolites like IPA. Future research may focus on comprehensive studies to ascertain further mechanisms at play and explore broader applications in the context of immunotherapy.