Research conducted by scientists in March 2025 has unveiled the crucial role of CLPB, or caseinolytic peptidase B protein homolog, in maintaining mitochondrial health, revealing its connection to various human diseases.
CLPB is the first identified mitochondrial disaggregase, a protein that assists in preventing harmful protein aggregation. This function is essential, as abnormal protein aggregates can disrupt cellular processes and contribute to a toxic cellular environment, which is evident in numerous diseases.
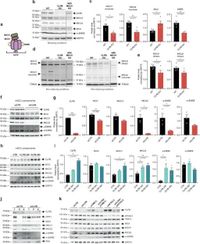
The study highlighted the interconnectedness of mitochondrial calcium signaling and the dynamics of mitochondrial fusion, showing that the loss of CLPB leads to significant impairments in both areas. Specifically, researchers demonstrated that when CLPB is absent, there is reduced mitochondrial calcium uptake and a decline in the activity of proteins crucial for mitochondrial fusion.
Utilizing advanced techniques such as immunoblotting, calcium imaging, and confocal microscopy, the team examined both standard laboratory models and patient-derived skin fibroblasts that carry mutations in the CLPB gene. This multifaceted approach revealed critical insights into how CLPB mutations might contribute to disease processes.
In experiments, the loss of CLPB resulted in alterations to essential components of the mitochondrial calcium uniporter (mtCU) complex, primarily MICU1 and MICU2. The functional impairment of these proteins hinders the mitochondria's ability to manage calcium levels effectively, directly impacting cellular metabolism and signaling.
Authors of the study stated, "CLPB is required to maintain mitochondrial calcium signalling and fusion dynamics." Their findings underscore that disturbances in mtCU and mitochondrial fusion activity due to CLPB loss could contribute to the pathology observed in patients with CLPB variants.
Additionally, the research showed that different mutations within the CLPB gene lead to variations in mitochondrial structure and function among patients. For instance, fibroblasts from patients with specific CLPB mutations exhibited distinct patterns of altered protein abundance and aggregation, correlating with known clinical manifestations.
With the growing recognition of mitochondrial dysfunction's role in a range of human diseases, this research highlights the importance of further exploring CLPB's role as a potential therapeutic target. Disruptions in mitochondrial calcium signaling and fusion dynamics may not only pertain to diseases already linked to CLPB but could also extend to other conditions characterized by mitochondrial dysfunction, such as neurodegenerative diseases.
Through this comprehensive investigation, researchers encourage a deeper understanding of CLPB's multifaceted roles in mitochondrial biology and its implications for disease mechanisms. This work lays the groundwork for future studies aimed at uncovering novel therapeutic approaches that might enhance mitochondrial health by targeting the pathways affected by CLPB mutations.