The study of human skeletal development has long posed challenges for researchers, particularly in understanding the complex processes of endochondral ossification—the mechanism through which most load-bearing bones are formed. In a groundbreaking study published in March 2025, a team of researchers has made significant strides in this field by successfully generating SOX9+ sclerotomal progenitors from human pluripotent stem cells. These newly identified progenitors hold great promise for modeling key stages of human bone and cartilage development, paving the way for improved therapeutic options for skeletal disorders.
Endochondral ossification is a pivotal biological process that results in the formation of the skeleton during development in mammals. It begins with the condensation of mesenchymal progenitors, leading to cartilage formation, followed by vascular invasion, and ultimately results in the generation of bone tissue. In humans, conditions such as osteoarthritis and other skeletal disorders impact millions, emphasizing the necessity for advanced research models that accurately recapitulate these developmental processes.
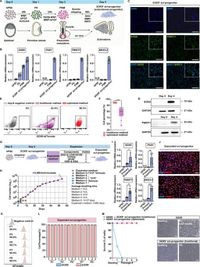
The research team aimed to establish a reliable model for studying human skeletal development, particularly given the limitations of traditional mouse models that often fail to capture human-specific factors. By deriving SOX9+ sclerotomal progenitors (or scl-progenitors) from human pluripotent stem cells, the researchers developed a specialized methodology for these cells that not only addresses the challenges of cell source heterogeneity but also ensures a robust pathway for osteochondral differentiation.
Using a lineage-specific induction protocol, the scientists successfully differentiated pluripotent stem cells into SOX9+ scl-progenitors with over 99% efficiency. This remarkable efficiency was achieved through a series of carefully designed steps that included the activation of genetic pathways crucial for sclerotomal specification. The resulting cells exhibited characteristics consistent with mesenchymal progenitors at the crucial pre-condensation stage of skeletal development.
In performing this research, the team observed that the SOX9+ scl-progenitors not only formed articular cartilage but also underwent spontaneous condensation—a critical early event in endochondral ossification. Moreover, these cells demonstrated the ability to progress through essential subsequent stages, including the formation of cartilaginous anlagen, chondrocyte hypertrophy, and vascular invasion, ultimately resulting in bone formation. The findings highlight the potential of these progenitors as vital tools for modeling human skeletal development.
Moreover, the study revealed the successful induction of structures resembling natural growth plates from the scl-progenitor-derived spheroids. These structures exhibited essential molecular and cellular traits consistent with those found in primary growth plates, marking a significant advancement in bioengineering approaches aimed at replicating human bone development in vitro.
The investigation also identified the specific integrin marker ITGA9, which plays a vital role in the isolation of SOX9+ scl-progenitors. This discovery enabled the researchers to develop a reporter-independent method for identifying these pivotal cells without needing to rely on genetically introduced markers, enhancing their applicability in research and potential therapeutic settings.
"Our findings present an integrated hPSC-based toolkit for modeling human skeletal development and bioengineering cartilage and bone tissues," wrote the authors of the article. This assertion emphasizes the broader implications of these advancements, particularly in developing treatments for cartilage injuries and degenerative diseases affecting the skeletal system.
In conclusion, this study provides a robust basis for future research into human skeletal disorders and developmental genetics. With the establishment of SOX9+ scl-progenitors, researchers can now more accurately investigate the mechanisms of human skeletal development and the potential mutations that lead to common bone disorders. As the scientific community continues to unravel the complexities of endochondral ossification, these findings pave the way for innovative approaches to regenerate and treat skeletal defects using advanced tissue engineering techniques.