Nairobi, Kenya - A recent nationwide serosurvey has revealed that Coxiella burnetii, the causative agent of Q fever, is prevalent among cattle in Kenya, with seropositivity rates found at 7.9%. This finding sheds light on an often-overlooked public health threat, particularly in low- and middle-income countries where surveillance capabilities are limited.
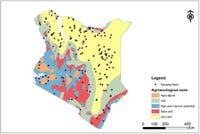
The research, conducted from November 2020 to August 2021, involved a comprehensive study of 6,593 cattle across various agroecological zones in Kenya. Researchers utilized a national cross-sectional survey method, collecting blood samples from randomly selected herds and analyzing them for seropositivity to C. burnetii using enzyme-linked immunosorbent assay (ELISA) techniques.
This study is significant as it marks the first extensive mapping of C. burnetii exposure in cattle across the country, underscoring the need for enhanced surveillance and control measures. The study's findings suggest environmental factors, such as soil type and wind speed, significantly influence the likelihood of C. burnetii exposure.
Specifically, the research identified that adult cattle were more likely to test positive compared to calves and younger animals. The study indicated that the odds of exposure were inversely related to the animal's age, with odds ratios of 0.24 for calves and 0.41 for weaners when compared to adults. Conversely, a one-unit increase in wind speed raised the odds of seropositivity by 1.27.
A striking discovery was the association between environmental variables and C. burnetii seropositivity. The presence of “petric calcisols,” a type of soil that contributes to the formation of dust and aerosols, was linked to higher seroprevalence rates. The research highlighted how environmental conditions can exacerbate the risk of pathogen transmission, particularly through aerosolized forms of the bacteria.
Dr. Ben Bett, a leading researcher on the study, stated, “Our findings illustrate the critical need for targeted interventions in regions identified as hotspots for C. burnetii exposure. Current vaccination protocols available in Europe cannot be implemented in Kenya, emphasizing the urgency for developing local strategies.”
Additionally, nearly a quarter of the cattle sampled (23.8%) were sourced from herds experiencing reproductive disorders such as retained placenta and abortion, which could also indicate higher exposure levels to C. burnetii. The prevalence was notably higher in herds reporting these issues (9.9%) compared to those without any reproductive disorders (7.2%).
Despite the challenges posed by C. burnetii, the One Health approach has been suggested as a framework for managing the disease through improved biosurveillance and educational efforts aimed at farmers and cattle handlers. Such integrated strategies could help mitigate the zoonotic risks posed by Q fever.
The results of this study add to the growing body of evidence highlighting the public health implications of C. burnetii in Africa. With the potential for outbreaks similar to those previously seen in countries like the Netherlands, where thousands of human cases emerged between 2007 and 2010, these findings underscore the need for immediate action and preventive measures.
As Kenya continues to face the challenges posed by emerging zoonotic diseases, the importance of refining existing health policies and enhancing inter-agency collaboration cannot be overstated.
In conclusion, this nationwide survey not only provides vital data on the prevalence of C. burnetii in Kenya but also opens avenues for future studies aimed at exploring its epidemiology within diverse livestock populations. With the backing of the government and health organizations, a concerted effort may mitigate the risks associated with this potent zoonotic pathogen.