Bacteria have evolved innovative immune systems to combat phage infections, one of the most prevalent threats to their survival. A recent study published on March 18, 2025, has unveiled the Hachiman system, a novel prokaryotic antiphage defense mechanism composed of HamA and HamB proteins. This groundbreaking research employs advanced structural biology techniques to elucidate how bacteria protect themselves against the relentless onslaught of bacteriophages, the viruses responsible for infecting bacterial cells.
The Hachiman system has been found to confer remarkable protection, with E. coli strains expressing this system showing defense capabilities ranging from 10- to 10,000-fold against various phages. This impressive range highlights the urgent need for improved anti-phage strategies as phage infections continue to rise globally, threatening not only individual bacterial populations but also agricultural and medical settings.
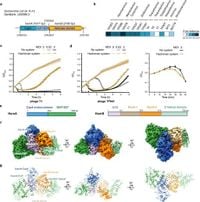
At the heart of the Hachiman system is the interaction between the proteins HamA and HamB. The study utilized cryo-electron microscopy techniques to determine the structure of the HamAB complex, achieving remarkable resolution at 3.1 Å. This allowed researchers to observe how HamA features a fusion of the Cap4 nuclease domain, playing a pivotal role in the biological defense mechanism. Structural analysis indicated the DUF1837 domain on HamA bears resemblance to the PD-(D/E)XK nuclease but is devoid of active sites, indicating its unique function within the immune response.
Through systematic research, scientists discovered HamA interacts with HamB to form the heterodimer HamAB. This complex mediates ATP hydrolysis, which is integral for executing DNA cleavage, effectively dismantling phage DNA and thwarting their replication within infected cells. Such cooperative functionality reinforces the importance of protein interactions underlying bacterial immunity. The authors stated, "HamA interacts with HamB to form the HamAB complex, which executes DNA cleavage for anti-phage defense." This collaboration between the two proteins emphasizes the evolutionary talent of bacteria to optimize immune responses against diverse threats.
The structural integrity of the HamAB complex revealed several protein interfaces, resulting from extensive hydrogen bonding and salt-bridge interactions. These interactions highlight the resiliency and adaptability of the Hachiman system. The findings suggest not only how this complex engenders effective phage resistance but also its potential as inspiration for novel antibacterial strategies.
Experimental results supported the defensive capabilities of the Hachiman system, demonstrating clear efficacy against various phages. Specific defense phenotypes were observed against known phages, such as Hanrivervirus and Warwickvirus, which displayed efficiency of plating reductions exceeding 1000-fold. Conversely, weaker protective effects were noted against phages like T7 and M13, illustrating the varying effectiveness of the Hachiman system depending on the type of phage encountered. The authors concluded, "The Hachiman system affords 10- to 10,000-fold protection against diverse phages." These discoveries situate the Hachiman system as one of many mechanisms bacteria employ to thrive within phage-rich environments, reinforcing the notion of 'defensive islands' within microbial genomes.
The broader significance of these discoveries cannot be understated. With phage therapy gaining interest as an alternative to traditional antibiotics, elucidations of systems like Hachiman may pave the way for engineered bacterial strains capable of enhanced phage resistance, influencing future treatments for antibiotic-resistant infections. The Hachiman system, found across nearly 2000 bacterial species and representing approximately 4% of bacterial genomes, signifies the microbial arms race continues, driven by constant pressure from phage challenges.
Future research is poised to explore the potential for manipulating the Hachiman system for biotechnological applications, especially as societies grapple with antibiotic resistance. By providing insights on the structural and functional interplay of the HamA and HamB proteins, this research not only sheds light on bacterial immunity but may lead to revolutionary advancements in microbial engineering.
Overall, the identification and characterization of the Hachiman defense system represent significant strides forward for microbiology and immunology, with tangible repercussions for health and medicine. The nuances of this complex's operations underline the exceptional adaptability of bacteria, thereby enhancing our perception of microbial life.