In the pursuit of understanding osteoporosis, one of the leading metabolic bone disorders impacting millions globally, scientists have unearthed significant insights regarding the regulatory mechanisms of a key gene known as WNT16. A recent study, published on March 20, 2025, sheds light on how WNT16 plays a crucial role in maintaining bone homeostasis through its interactions with nearby regulatory elements in the CPED1 gene.
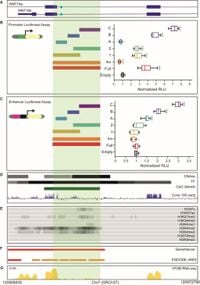
The WNT16 gene has long been associated with various skeletal phenotypes, a connection supported by numerous genome-wide association studies (GWAS). These investigations have identified distinct genetic loci linked to bone density; however, the functional implications of these associations have remained largely unexplored. The current research addresses this gap by dissecting the regulatory capacity of specific regions within WNT16, particularly intron 2, which appears to house crucial promoter activity vital for bone cell function.
Utilizing a methodology that included 4C chromatin conformation analysis and luciferase reporter assays, researchers performed their investigations on osteoblast-related cell types, including the human fetal osteoblast cell line hFOB 1.19 and the Saos 2 osteosarcoma cell line. The results revealed that various fragments obtained from the proximal part of WNT16 intron 2 physically interact with multiple putative regulatory regions within CPED1, suggesting a complex regulatory framework at play.
The study established that the WNT16 intron 2 region functions as an active promoter specifically within Saos 2 cells, amplifying the potential influence of this gene on bone density regulation. Furthermore, prior RNA sequencing data from hFOB cells indicated low expression levels downstream of the WNT16 promoter. This observation points to a novel understanding of how WNT16 is regulated, primarily through its interactions with distinct enhancer regions of CPED1.
"Our results suggest a novel regulatory mechanism of WNT16 in bone, mediated by physical interaction with various enhancer regions within CPED1," wrote the authors of the article. This statement encapsulates the essence of the study, marking a significant contribution to the landscape of bone biology and genetic research.
Previous GWAS have pointed towards the WNT16 gene as a prominent player influencing traits related to bone health, yet until now, the exact mechanisms underlying its activity remained largely uncharacterized. A key aspect of this research focused on verifying the functionality of a specific SNP, rs142005327, located within WNT16’s intron 2. By performing luciferase gene reporter assays, the researchers could delineate the promoter and enhancer activities associated with different constructs derived from intron 2.
The findings indicated that certain fragments, particularly B and C, exhibited substantial promoter and enhancer activities, outperforming other constructs in luciferase signal production. These insights have sparked new avenues for therapeutic strategies aimed at treating osteoporosis, underscoring the need for well-defined gene interactions in therapeutic contexts.
In exploring the underlying genetic framework, the researchers employed 4C-seq assays, generating libraries from their chosen cell lines. The assays enabled them to identify key regulatory interactions between WNT16 and CPED1, revealing previously unknown dynamics in the expression control of these genes.
Interestingly, the study observed that the alternative promoter region of WNT16 is physically interacting with CPED1, indicating a complex regulatory nexus. This connection is crucial, considering that previous findings suggested the impact of other genetic elements on osteoblast differentiation, further complicating our understanding of how these interactions might influence bone mass and strength.
The biological implications of these results are profound. Given the established link between WNT16 and bone mineral density (BMD), the current study illustrates how nuanced interactions between genes inform our understanding of bone health. The potential ramifications extend beyond mere genetics; they could illuminate new paths for osteoporosis treatment strategies that hone in on genetic expressions or modulate interactions at these regulatory junctions.
Future research will undoubtedly be pivotal in elucidating the fully operational spectrum of interactions governed by the WNT16 and CPED1 genes. The study opens the door for in vivo research, which might validate these findings across biological contexts. As highlighted in the conclusion, better understanding this regulatory interplay may eventually lead to innovative therapies aimed at improving bone health.
Within the yet-to-be-explored territories of genetic influence on bone material properties, the promise of WNT16 as a regulator introduces new prospects for interventions in metabolic bone diseases. With continued investigation into the molecular intricacies unveiled by this research, scientists may unlock the full potential of targeting these pathways to mitigate the impacts of osteoporosis and ensure stronger skeletal health.