Laryngeal squamous cell carcinoma (LSCC) is an aggressive malignancy that has seen a troubling increase in incidence rates in recent years. Up to 60% of patients diagnosed with LSCC are already in advanced stages of the disease, and unfortunately, the ten-year survival rates have shown a significant decline. Yet, recent research has unveiled potential pathways to improve diagnosis and treatment through the characterization of the tumor microenvironment (TME) and its interaction with various cell types.
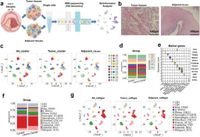
A recent study published on March 24, 2025, explores the cellular dynamics within the TME of LSCC using advanced single-cell RNA sequencing (scRNA-seq) techniques. This cutting-edge analysis has uncovered significant heterogeneity among tumor and immune cell populations present in LSCC. Notably, it has identified an increase in regulatory T cells at tumor sites and highlighted a particular macrophage subset known as Macrophage-C1-C1QC, which exhibited up-regulated expression of Peptidylprolyl Isomerase F (PPIF).
PPIF, a protein that plays crucial roles within cellular machinery, is integral to mitochondrial function and is associated with cancer progression through its influence on tumor metabolism and immune evasion. The study findings indicate that high expression of PPIF correlates with poor patient prognosis in LSCC, suggesting its potential as a therapeutic target.
Throughout the course of the research, a total of 40,667 cells from LSCC tissues were analyzed, revealing distinct populations that underscore the immune landscape of these tumors. For example, the presence of multiple clusters of CD4 and CD8 T cells, along with significant variations in macrophages, reflects the complex interplay of immune responses within the TME.
The researchers also found that the Macrophage-C1-C1QC subgroup, specifically, not only had enhanced expression of PPIF but also appeared to promote an anti-inflammatory phenotype that facilitates tumor growth. This relationship, notably through F11R-F11R signaling pathways, allows LSCC cells to evade cytotoxic actions of macrophages. Such findings emphasize the intricate mechanisms at play within the TME, demonstrating how LSCC tumors can manipulate immune responses to their advantage.
In addition to the immune cell dynamics, the study revealed that macrophage and malignant cells share common up-regulated genes, including those involved in immunosuppressive pathways. The authors elucidate how the up-regulation of PPIF in macrophages could lead to enhanced survival and immunosuppressive capacity, further facilitating LSCC progression.
This research not only sheds light on the critical role of PPIF but also opens doors to potential therapeutic strategies targeting macrophages and metabolic pathways in LSCC. For instance, leveraging inhibitors of PPIF might not only induce cancer cell apoptosis but also diminish the immunosuppressive environment that supports tumor survival.
In summary, the examination of PPIF and macrophage subtypes presents a dual approach to understanding and potentially mitigating LSCC. Insights derived from this study could significantly improve early diagnosis and treatment while also offering new perspectives for developing personalized therapies aimed at enhancing patient outcomes. Future research, especially involving larger patient cohorts, will be crucial in validating these findings and exploring the therapeutic potential of targeting PPIF in LSCC.