Researchers have identified two transcription factors, Foxk1 and Foxk2, as key multipliers of cardiomyocyte growth, providing an exciting avenue for cardiac recovery following injury. As ischemic heart disease remains a leading cause of mortality worldwide, the potential of endogenous cardiomyocyte proliferation presents a promising strategy for heart repair.
The expression of Foxk1 and Foxk2 decreases during postnatal development, coinciding with a significant reduction in the regenerative capacity of cardiomyocytes. However, studies show that overexpressing these factors can extend the window for proliferation in cardiomyocytes, enhancing the heart's capacity for repair after myocardial infarction (MI) injury. "Foxk1 and Foxk2 are potent inducers of cardiomyocyte proliferation and heart regeneration," wrote the authors of the article.
The research highlights how cardiomyocytes, the heart's muscle cells, undergo extensive death during MI, leading to damaged tissue that manifests as scarring. Unlike neonates, whose hearts can fully regenerate, adult hearts lose the ability to replenish cardiomyocytes efficiently. Consequently, when the myocardial tissue fails to regenerate, it leads to chronic heart failure and other serious health complications.
Throughout the study, scientists utilized adeno-associated virus serotype 9 (AAV9) to mediate the overexpression of Foxk1 and Foxk2 in mouse models. This included analyzing cardiomyocytes at various stages of development to understand how these transcription factors influence their ability to proliferate. Following this intervention, researchers noted significant improvements in heart regeneration capabilities. The compelling results indicate that Foxk1 and Foxk2 have a dual role in this process.
Firstly, these factors enhance cardiomyocyte cell cycle progression by directly activating CCNB1 and CDK1 expression, crucial components for cell division. By enhancing these pathways, cardiomyocytes can enter and progress through the cell cycle more efficiently, which is vital for regenerating heart tissue.
Secondly, the research highlights a metabolic shift induced by Foxk1 and Foxk2 that further supports cardiomyocyte proliferation. Both factors appear to promote glycolysis—an alternative energy-producing pathway—while suppressing oxidative phosphorylation. This metabolic reprogramming is essential for providing the energy and building blocks necessary for cell division. As stated in the article, "These findings establish Foxk1 and Foxk2 as promising therapeutic targets for cardiac injury."
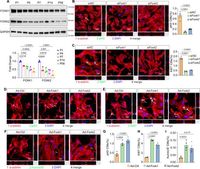
Through a series of immunostaining and biochemical analyses, researchers were able to quantify the proliferation rates of cardiomyocytes in both treated and untreated groups, providing compelling evidence of the enhanced proliferative capacity following Foxk1 and Foxk2 overexpression. The study utilized markers such as Ki67 and Aurora B to indicate active cell division, revealing that cardiomyocytes treated with Foxk1 and Foxk2 had significantly increased numbers of cells undergoing mitosis.
Further investigations revealed that the overexpression of Foxk1 and Foxk2 could counteract age-related declines in heart regeneration. These results have vital implications for developing new therapeutic strategies that mimic the regenerative potential of the neonatal heart in adults. This could potentially revolutionize treatment protocols for conditions like ischemic heart disease, where heart function is severely compromised.
While the potential for these transcription factors is promising, researchers cautioned the importance of carefully managing the treatment process. Rapid stimulation of cardiomyocyte proliferation and metabolic shifts could create imbalances that disrupt heart function. Therefore, future therapies must be optimized to ensure that increased cardiomyocyte turnover does not negatively impact cellular or tissue integrity.
Foxk1 and Foxk2 offer a remarkable framework for understanding cardiac repair mechanisms and open up exciting avenues for intervention in patients suffering from chronic heart diseases. Innovative gene therapy techniques that utilize these transcription factors could lead to groundbreaking approaches to enhance the heart's capacity to heal itself, potentially saving countless lives.
As the science of cardiac regeneration evolves, the incorporation of findings related to Foxk1 and Foxk2 may represent a pivotal step towards successfully treating heart conditions that currently lack viable solutions.