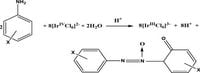
The oxidation of substituted anilines using iridium (IV) in aqueous perchloric acid represents a crucial advancement in understanding the kinetics of pollutant degradation. This study, recently published in Scientific Reports, highlights the complexities involved in the transformation of these ubiquitous environmental contaminants.
Research shows that the reaction exhibits a first-order dependence with respect to both the oxidant and the substrates involved. Specifically, electron-donating groups significantly enhance the reaction rate, whereas electron-withdrawing groups hinder it. The authors of the article noted, "The rate of oxidation reactions was decreased by the electron withdrawal effect from the benzene ring and increased by the electron-donating effect." This phenomenon was quantitatively assessed using the Hammett equation, which resulted in a negative reaction constant (ρ < 0) that increased with temperature, indicative of major changes occurring during the reactions.
The study meticulously evaluated a range of substituted anilines, including common variants like p-SO3H, p-COOH, and p-NO2, among others. Notably, the oxidation product was identified spectrophotometrically as 2-keto-azoxybenzene. This identification comes from the careful monitoring of the reactions using UV-visible spectrophotometry at a wavelength of 485 nm.
The authors also delved into the thermodynamics of the reaction, deriving an isokinetic temperature from different analytical plots: the Van’t Hoff plot resulted in a consistent isokinetic temperature of 317 K, while the Exner plot yielded an unexpected value of 439.32 K. This discrepancy suggests that enthalpy factors substantially govern the reaction rate within the studied conditions, an important insight into the underlying mechanics of these transformations.
As substituted anilines are often used in industrial applications, their environmental persistence poses significant risks. This research underscores the need to develop effective strategies for remediation, particularly regarding their entry into aquatic systems through pesticides and other chemical uses.
In terms of methodology, the researchers varied iridium (IV) chloride concentrations between 0.2 and 1.0 × 10−4 mol dm−3, maintaining precise control over the hydrogen ion concentration while monitoring the kinetics of the reactions across a temperature range of 303 K to 323 K. This rigorous approach allowed for the observation of how different substituents affected the overall reactivity and mechanism of oxidation.
The kinetic results reveal a trend where the oxidation of anilines is enhanced by the presence of electron-donating substituents, while the influence of electron-withdrawing groups is deleterious. A linear relationship was successfully constructed using the Hammett correlation at 313 K, providing further evidence for the underlying effects of substituents on reaction dynamics. As stated by the authors, "The linear relationship between the contributions of entropy and enthalpy implies that the susceptibility of the thermal reaction of anilines to substituent effect would be inversed simply by altering the temperature."
The implications of these findings are far-reaching, not only contributing to the body of knowledge regarding the degradation of hazardous substances but also enhancing the understanding of electron transfer processes in chemistry. With an isokinetic temperature determined at 317 K, these experiments suggest a common transition state across the various reactions, implying that similar mechanisms govern their behavior under similar conditions.
In conclusion, this detailed kinetic study sheds light on the oxidation processes of substituted anilines, contributing important insights into their environmental fate and the potential for developing more effective remediation strategies against these pollutants. The ongoing challenge posed by contaminants in aquatic environments makes such research vital for future ecological health and safety.