Researchers have unveiled the intricate molecular mechanisms behind alginate lyases, enzymes crucial for breaking down alginates found in brown seaweed.
This study provides new insights into the catalytic action of these enzymes, bridging gaps in our understanding of their structure and function. With significant implications for both industrial applications and environmental sustainability, this research underlines the importance of alginate-utilizing organisms.
Alginates, polysaccharides composed of mannuronic and guluronic acid, are integral components of cell walls in brown algae and are highly sought after in various markets, including food and biomedical industries. The global alginate market is valued at approximately $900 million, with 45,000 metric tons of alginates produced annually. Their unique properties enable applications ranging from thickening agents in food products to wound dressings in medicine.
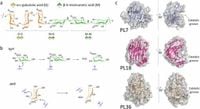
Despite their wide usage, the precise catalytic mechanisms of alginate lyases remained elusive due to the lack of structural data. This study employed advanced techniques—including time-resolved nuclear magnetic resonance (NMR), X-ray crystallography, and quantum mechanics/molecular mechanics (QM/MM) simulations—to explore the working of mannuronan-specific alginate lyases from polysaccharide lyase family 7 (PL7).
The researchers, led by a team from the marine fungus Paradendryphiella salina, provided atomic-level insights into how these enzymes interact with their substrates to facilitate alginate degradation. They demonstrated that the enzymes utilize a syn β-elimination reaction mechanism, where a single tyrosine residue acts both as a Brønsted acid and base during catalysis.
In their methodology, the researchers constructed high-resolution enzyme-substrate complexes and characterized the active site through neutron diffraction, revealing crucial interactions at the substrate-binding sites. The time-resolved NMR approach allowed for real-time tracking of product formation over an extended period, affirming that the enzymes predominantly produce oligomers with specific structural characteristics that favor their industrial utility.
This discovery resolves previous discrepancies regarding the mechanism of action among different alginate lyases, establishing that PL7 enzymes utilize a concerted but asynchronous mechanism for polysaccharide cleavage. Notably, the research highlights the enzyme's dependency on the protonation state of its key residues, with Tyr serving as a pivotal player in substrate interaction and bond cleavage.
As environmental concerns drive the search for more sustainable bioprocessing methods, these insights into alginate lyases are poised to propel further investigation into bioengineering applications that could enhance alginate utilization efficiency, leading to more targeted applications in various sectors.
Additionally, understanding these enzymatic processes can also inform the design of activity-based probes for identifying alginate lyases in diverse microbial habitats, thereby shedding light on microbial roles in biogeochemical cycles.
The findings, published in a recent article in a prestigious scientific journal, signify not only a major advancement in enzyme biochemistry but also the potential for transformative approaches in managing alginates across different industries.