The study of bacterial growth mechanisms has taken an intriguing turn with the discovery of a crucial protein known as StlP in filamentous actinobacteria, particularly in the species Streptomyces coelicolor. This stomatin-like protein is vital for orchestrating polar growth, particularly under hyperosmotic stress, conditions that challenge bacterial survival.
Cell walls are fundamental to bacterial integrity and protection against environmental stressors. They allow for growth through the insertion of new cell wall materials at specific sites. Actinobacteria exhibit a notable growth pattern called polar growth, where this insertion predominantly occurs at the cell poles, a mechanism diverging significantly from other rod-shaped bacteria like Escherichia coli. Understanding how this process operates under stress, such as high salt concentrations, has profound implications for both ecological fitness and potential medical interventions.
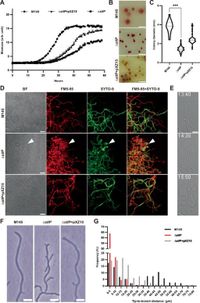
This recent research reveals how StlP plays a pivotal role in maintaining cell integrity when faced with osmotic stress. The absence of StlP in mutant strains resulted in an abnormal branching of filaments and compromised cell wall synthesis, leading to significant thinning of the cell wall. Specifically, it was noted that "the absence of StlP leads to branching of filaments, aberrant cell wall synthesis, thinning of the cell wall, and the extrusion of cell wall-deficient cells at hyphal tips," wrote the authors of the article.
StlP's primary function appears to be the formation of membrane microdomains that are crucial for ensuring that cell wall synthesis remains localized at the hyphal tips. This localization is essential for the radial expansion of the bacterial colony and for effective growth under adverse conditions. "StlP confers a competitive advantage to actinobacteria encountering hyperosmotic stress," the authors observed, highlighting its importance to the survival of these bacteria.
The investigation included generating mutants lacking the stlP gene and assessing their growth in media with varying osmotic pressure. Notably, the mutant strains exhibited significantly reduced growth rates compared to their wild-type counterparts under hyperosmotic conditions, underscoring the functional importance of StlP. For instance, while normal strains flourished, the stlP mutants exhibited poor growth and formed numerous wall-deficient cells, indicating a clear link between StlP and cell wall integrity.
Through advanced imaging techniques, researchers were able to visualize these findings, revealing that the StlP protein enriched membrane fluidity at hyphal tips. When StlP was reintroduced into these mutant strains, researchers observed a restoration of normal growth patterns and a reduction of wall-deficient cell extrusion, demonstrating that "the introduction of StlP enhanced polar growth and resilience in actinobacteria lacking this protein," according to the study's findings.
This discovery not only sheds light on the mechanisms of growth regulation in actinobacteria but also underlines the conservation of this protein across similar species, suggesting that StlP's role may be a fundamental aspect of bacterial survival in fluctuating environments. A broader analysis found that StlP is commonly present in various filamentous actinobacteria, indicating potential evolutionary adaptations enabling these organisms to thrive despite environmental challenges.
Importantly, these findings have significant implications beyond basic science. StlP's role in controlling the morphology of these bacteria suggests it could be a novel target in the development of antibacterial strategies, especially in pathogenic bacteria that can vary their growth patterns and potentially evade treatments targeting traditional pathways of cell wall synthesis.
In summary, StlP serves a crucial role in the polar growth of Streptomyces coelicolor by facilitating the necessary membrane organization and fluidity that supports proper cell wall synthesis. Such insights not only deepen our understanding of bacterial physiology but also open avenues for future research aimed at controlling pathogenic bacteria's growth by targeting fundamental aspects of their cell wall biosynthetic machinery. As StlP continues to unfold its secrets, it provides a fascinating glimpse into the intricate dance of bacterial survival and adaptation in an ever-changing environment.