A new clinical trial has demonstrated that Ulinastatin (UTI) significantly reduces the incidence of severe oral mucositis, a painful side effect of radiotherapy, in patients with locoregionally advanced nasopharyngeal carcinoma (LA-NPC). Conducted in China, the study reveals promising results for one of the most toxic effects of cancer treatment.
Oral mucositis is a common complication affecting around 50% to 100% of patients undergoing radiotherapy for nasopharyngeal carcinoma, and can lead to severe forms of the condition in about 30% to 50% of cases. It causes significant discomfort, increases the risk of malnutrition, and complicates ongoing treatment efforts. The design of this phase 3 trial sought to evaluate Ulinastatin's efficacy in preventing and treating radiotherapy-induced oral mucositis (RTOM) in LA-NPC patients.
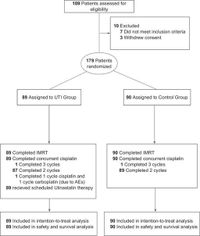
The trial, involving 179 patients from five centers between January 2018 and December 2021, assigned participants randomly to two groups: one receiving Ulinastatin treatment in addition to concurrent chemoradiotherapy, and a control group receiving only chemoradiotherapy. Key findings show that the incidence of grade 3 or higher RTOM during treatment was significantly lower in the Ulinastatin group at 25.8%, compared to 41.1% in the control group, demonstrating an effective preventative measure against this troubling side effect.
According to the authors of the article, the results indicate that, "Ulinastatin can effectively reduce the severity of RTOM and oral pain without increasing toxicity and compromising survivals." The study found that the duration of grade 3 RTOM did not differ significantly between the two groups, but recovery rates were notably higher in the UTI group, with 39.1% recovering compared to just 10.8% in the control group.
In addition to its impact on RTOM, Ulinastatin also showed a significant reduction in severe oral pain, with the incidence in the UTI group at 22.5%, while it was at 36.7% for the control group. This suggests that Ulinastatin may not only alleviate mucositis but also enhance the overall quality of life for patients enduring cancer treatment.
Long-term follow-ups indicated that survival rates after three years remained comparable between the two groups, with the Ulinastatin group's overall survival at 96.6% versus 94.4% for the control. This finding implies that Ulinastatin’s beneficial effects do not come at the cost of compromising overall survival, aligning with the trial’s safety observations where no significant adverse side effects were reported.
Ulinastatin, a refined glycoprotein derived from human urine, acts as a protease inhibitor and has shown in prior studies to have anti-inflammatory properties that could modify inflammatory responses in mucosal tissues. These findings suggest a prospective direction in managing radiotherapy-induced complications, especially in head and neck cancers.
Despite these advancements, the study does highlight the need for optimizing the dosage and administration frequency of Ulinastatin to enhance its convenience and effectiveness for clinical practice. The authors noted that as it stands, the protocol involves frequent intravenous administration which may pose challenges in routine patient care.
In summary, this phase 3 trial presents compelling evidence that Ulinastatin can serve as a viable option to mitigate the debilitating effects of radiotherapy in nasopharyngeal carcinoma patients. Further exploration into optimizing treatment timing and delivery methods is warranted to enhance patient comfort and treatment adherence.