Hereditary pheochromocytomas (PC) and paragangliomas (PG) arise from the neuroendocrine system and are notable for their potential to metastasize, complicacy whereby only 10-20% of patients exhibit metastatic progression. A recent study conducted by the A5 International Research Consortium has undertaken the first comprehensive genomic analysis of SDHB mutations within these tumors, shedding much-needed light on their clinical behavior and biochemical pathways.
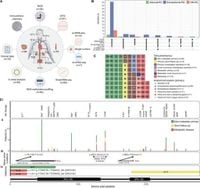
With multi-omic analysis of 94 tumors from 79 patients, researchers have begun to unravel the complex genetic architecture of these rare neoplasms. The study, published by authors of the article, highlights significant molecular markers and genomic alterations associated with metastatic disease and treatment response, as well as potential mechanisms of therapy resistance evident via alterations in genes like TERT and ATRX.
PC and PG are commonly linked with excess catecholamine production, which poses serious health risks including cardiovascular issues. The incidence rate between non-metastatic and metastatic tumors varies drastically, with the risk for metastasis markedly increased among those carrying pathogenic variants of SDHB. Despite the advancements, prognosis remains challenging due to the inability of current biomarkers to reliably predict disease progression.
Within their intriguing findings, the authors highlight genomic alterations indicative of increased mutation load, particularly surrounding TERT and ATRX genes, as these constructs are strongly correlated with metastatic behavior. Such exploration paves the way for refining patient surveillance strategies based on molecular characteristics, potentially altering clinical management standards.
The researchers utilized combined methodologies, leveraging whole-genome sequencing (WGS), whole transcriptome sequencing (WTS), and DNA methylation profiling—approaches previously underutilized to elucidate the full genomic spectrum of these tumors. Previous genomic studies often relied on whole-exome sequencing, which limited the investigative capabilities to capture non-coding variants and structural changes, aspects now addressed through this comprehensive multi-faceted approach.
Somatic mutations observed within the study included TERT and ATRX alterations, prevalent within the metastatic cohort, highlighting their role as secondary drivers of metastatic progression. Importantly, this elevated mutation burden aligns with findings from previous literature, emphasizing the potential for incorporating genetic insights as prognostic biomarkers.
Notably, distinct transcriptional patterns were found to correlate with tumor aggression. The authors note how genomic profiling can identify patients at higher risk for progressive disease—insights which significantly augment the clinical approach to care.
Given the inherited propensity of SDHB mutations for oncogenic transformation and metastasis, the implementation of precision medicine becomes increasingly pertinent. The identification of syndromes linked to hereditary endocrine neoplasms creates future avenues for screening, prevention, and therapeutic intervention strategies.
Resistance to conventional alkylator therapies was another focal point uncovered by the research. The study illuminated mechanisms such as MGMT overexpression and mismatch repair deficiency, significant contributors to treatment failure. These revelations align with similar findings across oncological studies investigating mechanisms of resistance related to tumor metabolism and genomic integrity.
Highlighting the significant mutation load and associated TERT/ATRX genomic alterations, the research provides compelling evidence of their potential role as predictive biomarkers for therapeutic response and metastatic risk. Insights gleaned from multi-omic profiling are anticipated to transform future clinical outcomes for patients burdened with hereditary pheochromocytomas and paragangliomas.
It is evident from the study led by the A5 consortium, as summarized, the genomic complexity of SDHB-deficient tumors showcases not only their unique molecular signatures but also necessity for continued research to develop effective predictive models for treatment and prevention. By integrating genomic advances with clinical practice, researchers anticipate fostering improved patient stratification and outcomes.