Acute myeloid leukemia (AML), particularly the MLL-rearranged subtype (MLLr AML), continues to pose significant therapeutic challenges due to its aggressive nature and high rates of relapse. Recent advances have focused on inhibiting the menin-MLL interaction, yet resistance often arises swiftly, complicating treatment efforts. A new study from researchers at Baylor College of Medicine explores an innovative strategy that could enhance the efficacy of menin inhibitors through targeting metabolic pathways.
The study reveals that leukemia stem cells (LSCs) exhibiting MLL rearrangements rely heavily on guanine nucleotide biosynthesis. Inhibiting this metabolic pathway not only sensitizes these cells to menin inhibitors but also promotes their differentiation into mature myeloid cells. This differentiation is a critical step in combating AML's typical resistance to therapeutic agents, suggesting a two-pronged approach to treatment could be feasible.
Acute myeloid leukemia arises from the transformation of hematopoietic stem and progenitor cells and is marked by a diverse genetic landscape. MLLr leukemias are particularly notorious for their poor prognosis, reflected in dismal five-year survival rates that necessitate the exploration of new treatment modalities.
The research points to a crucial role for inosine monophosphate dehydrogenase 2 (IMPDH2) in maintaining cellular levels of guanine nucleotides. By pharmacologically inhibiting IMPDH2 in MLLr AML cells, the researchers observed reduced levels of guanine nucleotides, which are vital for the cells’ survival and proliferation. This inhibition led to increased expression of differentiation markers, hence pushing the LSCs towards a more mature state.
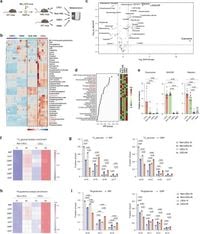
In the study, the team measured the abundance of metabolites associated with purine biosynthesis in MLL-AF9 driven murine AML models and found significant enrichment in LSCs. They used techniques such as liquid chromatography-mass spectrometry and isotope tracing to establish that modifications in nucleotide levels influenced cellular behavior and gene expression.
The researchers wrote, "Our findings underscore the requirement of guanine nucleotide biosynthesis in maintaining the function of the LEDGF/menin/MLL-fusion complex and provide a rationale to target guanine nucleotide biosynthesis to sensitize MLLr leukemias to menin inhibitors." This underscores the intricate link between metabolism and oncogenic signaling pathways.
Further experiments illustrated that blocking guanine biosynthesis not only hindered the MLL-fusion complex's ability to bind chromatin but also increased the activity of transcription factors associated with myeloid differentiation. These findings open up a potential for combination therapies that could first induce differentiation of leukemic cells and then target them with menin inhibitors, possibly leading to improved treatment outcomes.
Another important finding involved the use of well-established drugs like mycophenolate mofetil (MMF). MMF is traditionally used as an immunosuppressant but demonstrated a remarkable capability to enhance myeloid differentiation in vitro, thereby showcasing its potential repurposing as a cancer therapeutic. Notably, guanosine supplementation could reverse the differentiation effects, indicating a tight regulation of the purine nucleotide pathway.
Moreover, the study showed that the MLLr AML cells displayed heightened sensitivity to guanine biosynthesis inhibitors, particularly when treated in conjunction with menin inhibitors. The combination led to pronounced reductions in leukemic cell proliferation and enhanced differentiation into mature myeloid cells.
The findings suggest that metabolic interventions, particularly targeting the guanine nucleotide biosynthesis pathway, may provide a strategic advantage in treating MLLr AML. This approach could not only help overcome the rapid development of drug resistance seen with current therapies but also contribute to the overall effectiveness of AML treatment strategies.
As the authors concluded, “Inhibition of guanosine production promotes myeloid differentiation, suggesting that modulation of the guanosine pathway either through inhibition or overactivation may drive AML differentiation.” This study provides a foundational understanding that can inform next-generation treatments and combo therapies aimed at achieving long-term remission for patients suffering from this challenging malignancy.
In summary, while MLLr AML remains a formidable foe in oncology, the insights from this study pave the way for novel strategies that embrace both metabolic targeting and traditional inhibitory approaches to extend survival and improve the quality of life for affected patients.