In a significant advancement for dermatological research, scientists have uncovered that deficiency in steroid sulfatase (STS) plays a pivotal role in enhancing keratinocyte differentiation, a finding with critical implications for understanding and potentially treating skin disorders, particularly X-linked ichthyosis (XLI). This genetic disorder is characterized by severe hyperkeratinization, resulting from STS deficiency that disrupts the conversion of steroid sulfates into their active forms.
The study, conducted by researchers at Chung-Ang University and published on March 21, 2025, demonstrates how STS deficiency significantly amplifies calcium signaling pathways within skin cells, further driving excessive keratinocyte differentiation. Keratinocytes are the primary cell types found in the epidermis, and their proper functioning is crucial for maintaining skin health.
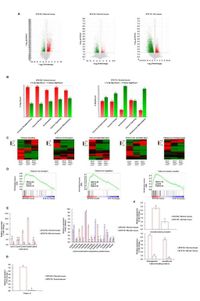
Research findings revealed that STS-deficient cells exhibited a markedly increased intracellular calcium influx, suggesting that these cells became more sensitive to calcium—a critical regulator of cell differentiation. The analysis included advanced techniques like RNA sequencing conducted on skin tissues from both STS knockout (KO) and transgenic (TG) mouse models, which revealed substantial upregulation of genes associated with calcium signaling and keratinocyte differentiation.
Notably, the increase in calcium influx may be attributed to significantly elevated levels of calcium-sensing receptors (CasR) present in STS-deficient keratinocytes. This observation indicates possible pathways through which the skin cells can unintentionally amplify differentiation signals in the absence of adequate STS activity. “Reduced STS expression and inhibition of its activity enhance calcium responsiveness, induce CasR expression, and amplify calcium signaling, thereby promoting keratinocyte differentiation,” the authors wrote.
In experiments utilizing the STS inhibitor STX-64, researchers found that this treatment led to a significant dose-dependent increase in CasR expression. Further investigations showed increased expression levels of early differentiation markers such as keratin 1 and keratin 10, both integral to proper skin formation and function. By inhibiting STS, researchers were able to observe enhancements in marker expression that suggest therapies could be developed to mitigate the symptoms of XLI.
The implications of these findings extend beyond merely understanding STS deficiency’s role in XLI. They provide critical insights into how skin differentiation could be modulated through calcium pathways, presenting new avenues for therapeutic interventions. Furthermore, STS deficiency not only upregulates early differentiation markers but also enhances the expression of terminal differentiation markers like involucrin and loricrin in keratinocytes, thus indicating a comprehensive impact of STS on skin properties.
As the study noted, “Collectively, our findings demonstrate that STS deficiency amplifies calcium signaling and accelerates keratinocyte differentiation,” highlighting the dual pathways involved in skin barrier function disruption associated with STS deficiency. Moreover, heightened calcium signaling in STS-deficient keratinocytes may lead to severe keratinization, contributing to hypopigmentation observed in the disorder. However, the study concludes by stressing the need for future exploration into the precise mechanisms guiding how STS regulates expression of CasR, paving the way for more targeted approaches in skin disease treatment.
The research not only sheds light on the mechanics of skin disorders like XLI but also points toward the broader implications of calcium and STS interactions in cellular signaling and differentiation processes. Such understanding reinforces the idea that managing calcium levels could present novel strategies for improving skin health and tackling keratinization-related diseases in clinical settings.
In conclusion, this research offers a promising direction for both therapeutic development and our understanding of the cellular processes governing skin conditions that stem from genetic factors. Altering calcium signaling pathways through interventions at the level of STS activity could become a viable strategy for tackling X-linked ichthyosis and similar disorders in the future.