A new study reveals that papillary renal cell carcinoma (pRCC) is driven by disruptions in the Hippo/YAP signaling pathway, presenting a significant opportunity for targeted cancer therapies. Researchers have discovered that hyperactivation of the YAP1 protein can lead to the transformation of renal epithelial cells, driving the development of this common yet challenging form of kidney cancer.
pRCC accounts for approximately 15% of all kidney cancers, and its mechanisms remain under-explored, which contributes to the limited treatment options available today. The Hippo signaling pathway, a key regulator of organ size and cell growth, has recently been implicated in various cancers, but its precise role in pRCC had not been fully understood until now.
To unravel the complexity behind pRCC, a team of researchers employed a transgenic mouse model, engineered to express a constitutively active form of YAP1. This model allows for the observation of pRCC progression and the dissection of the underlying molecular mechanisms, contributing significantly to cancer research.
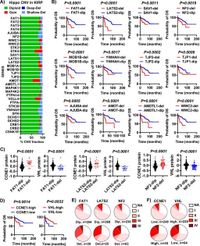
The study demonstrated that hyperactivation of YAP1 led to the dedifferentiation of renal tubular epithelial cells and significant renal overgrowth, evident within days of the manipulation. By using single-cell RNA sequencing analysis, the research team identified that changes in YAP1 levels lead to not just cell transformation but also to the accumulation of myeloid-derived suppressor cells (MDSCs), which further promotes tumor development.
As the study noted, "Our results identify the disrupted Hippo/YAP signaling as a major contributor to pRCC and suggest that targeting the disrupted Hippo pathway represents a plausible strategy to prevent and treat pRCC," wrote the authors of the article. This insight casts new light on potential therapeutic approaches that could mitigate pRCC progression by directly targeting the YAP1 signaling pathway.
Research showed that the frequent shallow deletions of key components of the Hippo pathway, specifically in tumor suppressors such as NF2 and YWHAH, are prevalent in pRCC cases. These deletions did not lead to full gene loss, but rather to a functional impairment that promotes YAP1 activity. This observation hints at a potential new preventative strategy in pRCC, focusing on the functional restoration of the Hippo signaling.
Following the identification of the critical role of YAP1, the researchers emphasized the significant role of MDSCs in facilitating the tumor's immunosuppressive environment, which can help tumor cells evade the immune response. This underscores the importance of understanding the interaction between YAP1 hyperactivation and immune evasion mechanisms in pRCC.
The study's implications are far-reaching, as it not only highlights YAP1's role in initiating and promoting renal tumors but also positions MDSCs as a promising target for therapeutic intervention. Current treatment strategies for pRCC remain limited, making this research particularly timely and relevant.
In conclusion, the disruption of the Hippo/YAP signaling pathway opens avenues for innovative therapeutic strategies, as targeting this pathway may effectively address the root causes of pRCC and improve patient outcomes. Future studies will be critical to thoroughly explore the pharmacological interventions targeting YAP1 and MDSCs, with the hope of translating these findings into viable clinical options.