In a groundbreaking study published in Nature Communications, researchers have uncovered the role of Septin5 in regulating insulin secretion in pancreatic beta-cells. This discovery highlights how the elimination of Septin5 enhances the hormone's release, potentially paving the way for new treatments for type 2 diabetes (T2D).
The research team found that Septin5 is abundant in both rodent and human beta-cells, where its deletion significantly increases biphasic glucose-stimulated insulin secretion. Such a boost in insulin levels could be crucial for individuals with T2D, who often struggle with glucose regulation due to insufficient insulin release.
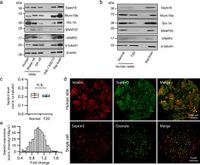
Septin proteins, known as a highly conserved family of GTP-binding proteins, play vital roles in various cellular functions, including secretion and cell division. Septin5 specifically has been previously implicated in neurotransmitter release but its contribution to insulin secretion remained unclear until now. Using both Septin5 knockout (KO) mice and human donor islets, the researchers demonstrated that the absence of Septin5 improved insulin secretion, with increases of 137% in the first-phase response and 122% in the second-phase response compared to wild-type (WT) controls.
Super-resolution imaging revealed that Septin5 is preferentially organized at microtubule-plasma membrane contact sites, which provides crucial anchoring points for secretory granules. The researchers found that deletion of Septin5 not only increased the dynamics of insulin granules but also improved their access to the plasma membrane, enabling better fusion with calcium channels needed for insulin release.
The study also featured analysis of the complex dynamics involved in insulin granule mobilization. When Septin5 is removed, insulin granules that had previously been tethered to microtubules are freed, enhancing their potential to interact with calcium channels during the secretion process. This finding is particularly relevant as it corrects defects observed in the insulin release mechanisms of T2D patients.
Dr. F. Kang, one of the authors of the study, explained that "Septin5 deletion increases the mobility of insulin SGs and facilitates their access to the plasma membrane by regulating cortical microtubule stability." This observation suggests that Septin5 functions somewhat like a brake on insulin secretion, and its absence allows for a more efficient release process.
Furthermore, the research team showed that this enhancement of insulin secretion was not merely theoretical; using adeno-associated viral vectors to deliver Septin5-shRNA into human islets led to a striking 46% increase in first-phase secretion and an impressive 82% increase in the second phase during perifusion assays.
The implications of these findings could be significant for diabetes treatment strategies. By targeting Septin5 for inhibition, it may be possible to develop new therapeutics that enhance insulin secretion and help normalize blood sugar levels in people suffering from T2D.
Notably, earlier studies have indicated that impaired coupling of insulin granules to calcium channels is a characteristic of T2D. However, this new study found that the enhanced secretion observed with Septin5 depletion resulted in increased clustering of insulin granules on L-type calcium channels—points of critical calcium influx for the exocytosis of insulin granules.
In T2D beta-cells, this coupling is often disrupted, leading to insufficient insulin release. The study highlights how improving this interaction through Septin5 deletion could rectify some of these dysfunctions.
The researchers conclude that their findings provide compelling evidence that inhibiting Septin5 could enhance the efficiency of insulin secretion under both normal and pathological conditions, suggesting new avenues for treatment strategies aimed at improving glucose tolerance and managing diabetes effectively.