Scientists have successfully unraveled critical dynamic processes in the assembly of colloidal crystals using an innovative approach that highlights their potential applications in various fields, from optoelectronics to biosensing. A team of researchers conducted a groundbreaking study on the convective self-assembly of polystyrene nanospheres (PS NSs) using in-situ small-angle X-ray scattering (SAXS) and ex-situ scanning electron microscopy (SEM) to gain insights into the real-time formation of nanostructures.
The dip-coating technique employed in the research allows a substrate to be immersed in a colloidal suspension. As the substrate is withdrawn, evaporation occurs, leading to the assembly of particles at the three-phase contact line where air, liquid, and substrate intersect. "This is a new era of understanding self-assembly mechanisms and how we can better control them," wrote the authors of the article.
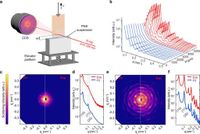
The study, published in Nature Communications, highlights how the researchers were able to observe the assembly processes at the gas-liquid-solid interfaces, revealing the sequential formation of monolayers that serve as templates for subsequent multilayers. Utilizing a dip coater, the mica plates were immersed 30 mm deep into a PS NS solution and lifted at a constant withdrawal speed of 0.1 mm/min, while parameters such as environmental temperature (maintained at 27 ± 1 °C) and relative humidity (55% ± 5%) were carefully controlled.
At specific heights during the dip-coating process, the SAXS measurements revealed significant findings. For instance, at H=3800 μm, the formation of a two-dimensional hexagonal monolayer was indicated by three Bragg peaks observed in the SAXS patterns at q = 0.054, 0.094, and 0.108 nm-1, consistent with theoretical predictions for hexagonal lattices. The meticulously recorded patterns demonstrated that the 2D hexagonal structure was highly ordered, setting the stage for the subsequent multilayer formation.
Once the multilayer was deposited, the researchers noted that its structural quality was closely correlated with that of the initial monolayer. In fact, the final multilayer structure achieved was identified as a four-layer face-centered cubic (fcc)-type stacking, with a lattice constant measured at 127.2 nm. The precise formation of these layers depended on the effective interfacial template provided by the monolayer.
Numerical simulations conducted during the study also illustrated the underlying mechanics of evaporation and fluid dynamics, which play crucial roles in the assembly process. The authors noted that the evaporation rate, found to follow the theoretical relation F(H) = a(5000-h)b, peaked at the contact line, instrumental for effective particle transport to the assembling front. The results indicated that the average flow velocity increased substantially as meniscus thickness decreased, with readings of approximately 40 μm/s at H=3800 μm.
This dynamic system represents a significant advance in the comprehension of how colloidal particles achieve ordered structures without reaching thermodynamic equilibrium. Further experiments showed that adjustments in environmental conditions such as temperature impacted the quality of both the monolayers and multilayers formed, with the best ordering obtained at 27 °C.
The research findings suggest that the principles of this self-assembly process could extend to various colloidal materials, especially those exhibiting similar size, charge, and dispersion behaviors. Given the considerable potential for applications involving the dip-coating technique in developing advanced micro- and nanostructured films, these insights into assembly mechanisms will aid researchers in optimizing methods for producing materials with tailored properties.
As the field of colloidal self-assembly progresses, studies like this one provide a vital stepping stone toward fully harnessing the capabilities of nanoscale materials. Understanding the interplay of kinetic and environmental factors in active assembly processes is essential for advancing numerous applications across diverse scientific disciplines.