Researchers have introduced a promising new approach to treating Parkinson's disease (PD) by targeting the underlying mechanisms of the disorder through a synthetic peptide named AmyP53. This peptide demonstrates efficiency against the harmful effects of the protein alpha-synuclein, which is known to play a significant role in the pathology of Parkinson's disease.
Parkinson's disease is characterized by the loss of dopaminergic neurons, leading to various motor and non-motor symptoms. Currently, the treatments available focus primarily on managing dopamine-related issues but do not modify the disease's progression. The proposed strategy of utilizing AmyP53 offers hope for addressing the root cause of the disorder.
Previous studies have shown the protein alpha-synuclein's intimate connection with PD, as it forms neurotoxic oligomers when interacting with lipid membranes. The researchers propose to interrupt this process by using AmyP53 to inhibit the harmful oligomer formation. This peptide is derived from the ganglioside-binding domains of both alpha-synuclein and the Alzheimer's beta-amyloid protein, effectively allowing it to compete for the same binding sites as alpha-synuclein, thereby preventing oligomerization.
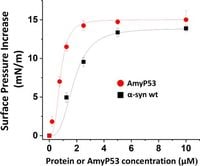
AmyP53 demonstrates intriguing properties when pitted against both wild-type and mutant forms of alpha-synuclein, including variants associated with genetic predispositions to Parkinson's disease. Laboratory tests revealed AmyP53’s capacity to bind to ganglioside membranes swiftly and effectively, emphasizing its potential therapeutic benefits.
One of the key features of AmyP53 is its adaptability; it can change conformations when binding to gangliosides, which enhances its binding efficiency. The studies conducted indicated significantly lower concentrations of AmyP53 were required to initiate these interactions compared with alpha-synuclein, showcasing its high affinity for gangliosides and the increased safety profile it presents.
The peptide was evaluated using aged brain cell models which simulate the environment of neurons affected by PD. Results showcased AmyP53’s ability to prevent the onset of calcium influx typically induced by alpha-synuclein oligomers, highlighting its potential neuroprotective properties.
Further validation was done using animal models of Parkinson's disease. When administered intranasally, AmyP53 showed no adverse side effects and successfully mitigated dopaminergic neuron loss caused by alpha-synuclein protofibrils. This indicates not only its safety but also its effective delivery route to the brain, allowing for prolonged therapeutic benefits.
Due to the unique way AmyP53 interacts with lipid rafts—a membrane structure associated with neural functions—it offers insights toward developing new neuroprotective therapies. According to the findings, targeting the gangliosides through this innovative approach could be the key to halting or reversing damage caused by neurotoxic proteins.
The study encapsulates groundbreaking findings which could pave the way for effective disease-modifying treatments for Parkinson’s disease. By focusing on the lipid-based interactions rather than merely on the proteins associated with neurodegeneration, this research could shift paradigms and inspire new therapeutic strategies for neurodegenerative conditions more broadly.