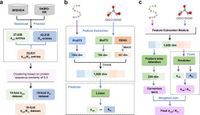
In the evolving field of synthetic biology, improving enzyme predictions is critical for enhancing the efficacy of industrial processes, from pharmaceutical development to biofuels. A recent study has introduced a transformative tool named CataPro, which leverages deep learning to advance the accuracy of enzyme kinetic parameter predictions significantly.
Enzymes serve as biocatalysts essential for a multitude of industrial applications, including the production of chemicals, pharmaceuticals, and food products. However, traditional models for predicting enzyme parameters such as turnover number (kcat), Michaelis constant (Km), and catalytic efficiency (kcat/Km) have suffered from issues of inaccuracy and overfitting, making it challenging to obtain reliable predictions in a timely manner. To address these limitations, the authors developed CataPro, a robust prediction model that utilizes deep learning combined with molecular fingerprints and pre-trained frameworks.
By constructing unbiased datasets drawn from established databases like BRENDA and SABIO-RK, the researchers ensured that CataPro was trained on a diverse range of enzyme-substrate data, effectively mitigating the risks of overfitting. "Compared with previous baseline models, CataPro demonstrates clearly enhanced accuracy and generalization ability on the unbiased datasets," wrote the authors of the article. This leap in performance not only signifies a technical advancement but also paves the way for more reliable enzyme applications across different sectors.
In one notable application, CataPro was employed in a representational enzyme mining project that identified an enzyme known as SsCSO, which exhibited an astonishing 19.53 times increase in activity over the original enzyme (CSO2). Following the predictions generated by CataPro, further engineering of SsCSO resulted in additional enhancements, achieving a notable 3.34-fold increase in enzymatic activity. The authors stated, "This reveals the high potential of CataPro as an effective tool for future enzyme discovery and modification." With these significant findings, the capabilities of CataPro are not only theoretical; they have real-world implications for enhancing enzyme functions critical in industrial settings.
CataPro's architecture incorporates pre-trained models that analyze amino acid sequences of enzymes coupled with molecular structure data of substrates. This comprehensive approach enables the model to predict kcat, Km, and kcat/Km with unprecedented accuracy by learning from a rich pool of training data while avoiding common pitfalls such as data leakage, which can skew results in other models.
The implications of these advancements extend beyond just academic research; they hint at a future where enzyme engineering is transformed by predictive analytics, allowing for streamlined processes in biocatalyst development. As industries seek to improve efficiency and sustainability, CataPro may be key in unlocking new enzymatic pathways and enhancing existing ones.
As the field of enzyme research continues to develop, the introduction of models like CataPro represents a paradigm shift in how enzymatic functions and activities can be predicted. This advancement not only facilitates deeper understanding but also allows for more extensive applications across several domains, from food safety to environmental sustainability.
To sum up, the introduction of CataPro marks a significant achievement in enzyme kinetic parameter prediction, showcasing its potential to revolutionize enzyme discovery and modification. With ongoing efforts to refine and expand its capabilities, the tool stands to play a crucial role in the synthetic biology landscape, promising efficient and effective solutions that meet industrial demands while adhering to modern ecological standards.