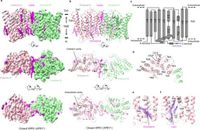
The regulation of inorganic phosphate (Pi), a vital component for life, has taken center stage in a recent study highlighting the intricate mechanisms of human cellular function. Researchers have characterized the cryo-electron microscopy (cryo-EM) structures of the human XPR1-KIDINS220 complex, revealing insights into how this complex controls phosphate export in human cells.
Phosphate is essential for various biological processes, including energy transfer, DNA synthesis, and cellular signaling. It is primarily absorbed in its inorganic form (Pi) and must be maintained within precise concentrations to prevent toxicity. The human Xenotropic and Polytropic Retrovirus Receptor 1 (XPR1) has been identified as the sole known phosphate exporter in vertebrates, making it crucial for cellular phosphate homeostasis. Dysfunction in this mechanism can lead to serious health issues, including ovarian cancer and primary familial brain calcification (PFBC).
This study employs advanced cryo-EM techniques to elucidate the structural dynamics of the XPR1-KIDINS220 complex in both its closed and outward-open conformations. The authors discovered that in the presence of inositol hexaphosphate (InsP6) and phosphate, XPR1 transitions to an outward-open conformation, which significantly indicates active phosphate export. As stated by the authors, "In the presence of inositol hexaphosphate (InsP6) and phosphate, the complex adopts an outward-open conformation, with InsP6 binding the SPX domain and juxtamembrane regions, indicating active phosphate export."
Structurally, XPR1 features various critical domains, including the transmembrane domain where the phosphate ion transport takes place. The study highlights how InsP6 interacts with the SPX domain of XPR1, facilitating its phosphate export function.
Combined with KIDINS220, a regulatory protein, the XPR1’s functionality is enhanced, allowing for the precise regulation of phosphate levels within the cell. These findings imply a synergy where InsP6 acts as a molecular glue, while KIDINS220 assists in maintaining structural integrity and enabling the transport process. The authors emphasize the potential for these insights to inform cancer therapies, stating, "These insights into phosphate regulation may aid in developing therapies for ovarian cancer." This connection highlights the broader relevance of fundamental biological research to clinical applications.
The methodology utilized in this comprehensive study—specifically, cryo-EM—provides significant advantages in structural biology by allowing for detailed visualization of protein complexes in their native states. The ability to elucidate the XPR1-KIDINS220 complex’s structure enables researchers to gain effective insights into how cells manage phosphate levels, which is pivotal for metabolic processes. The detailed understanding of this transport cycle opens avenues for new therapeutic strategies aimed at diseases linked to phosphate dysregulation.
Importantly, XPR1 and its associated pathways illustrate the delicate balance cells must maintain regarding phosphate ion levels. Mutations or dysfunctional behaviors in these transport mechanisms can lead to severe health issues, underscoring the importance of ongoing research in cellular transport processes. As scientists continue to untangle these mechanisms, the goal remains clear: to enhance therapeutic approaches to combat diseases influenced by phosphate regulation.
The findings from this study reveal a significant advancement in our understanding of how XPR1 and KIDINS220 collaborate to regulate phosphate export. By describing the structural and functional interactions within this complex, researchers provide a foundation for future investigations into phosphate regulation and the potential therapeutic applications of this knowledge in treating metabolic disorders and cancer.