The dynamics of mitochondria, the powerhouse of the cell, are pivotal for maintaining cellular health, a recent study has illuminated how a specific lipid, diacylglycerol (DAG), plays a critical role in this process. Utilizing an innovative inducible heterodimerization system, researchers demonstrated that localized production of DAG leads to rapid fragmentation of the mitochondrial network, a phenomenon mediated by key proteins known as endophilin B and dynamin-related protein 1 (Drp1).
Mitochondria are not static entities; they exist as dynamic networks, constantly undergoing cycles of fusion and fission. This continuous reshaping is essential for various cellular functions, including metabolism and organelle quality control. While much is known about the protein machinery that drives these processes, the influence of specific lipids on mitochondrial fission and fusion remained a less explored territory, particularly for DAG.
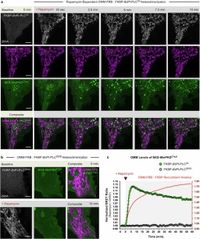
The research team found that the localized production of DAG triggered distinct changes in mitochondrial morphology. They noted, "Localized production of DAG initiates rapid and uniform fragmentation of the mitochondrial network in a Drp1-dependent manner," emphasizing how DAG levels could directly affect mitochondrial dynamics.
The research harnessed HEK293A and HeLa cells to study the effects of localized DAG production. Through precise molecular manipulation, they induced the creation of DAG at specific sites on the outer mitochondrial membrane (OMM) and monitored the resulting changes using advanced imaging techniques. The consequences were striking: the production of DAG facilitated the binding and assembly of both EndoB proteins and Drp1, underscoring the lipid’s crucial role in mitochondrial fission.
Furthermore, the study revealed that the presence of DAG not only promotes Drp1 activity but also activates endophilin B proteins, both of which are essential for efficient membrane remodeling. The authors concluded, "The presence of DAG directly facilitates the membrane binding and self-assembly of Drp1 as well as both isoforms of EndoB," illustrating how lipid interactions prompt cellular machinery to respond.
Through a series of experiments, the researchers demonstrated that altering lipid composition within the OMM was sufficient to significantly impact mitochondrial morphology, leading to fragmentation that had not been accurately accounted for in previous studies. They assert, "Together, our results reveal that OMM lipid composition directly affects mitochondrial morphology," thus filling a critical knowledge gap regarding the role of lipids in mitochondrial biology.
This discovery has broader implications for understanding metabolic diseases and age-related mitochondrial dysfunctions, as disrupted mitochondrial dynamics are central to these health issues. With DAG and its impact firmly on the scientific radar, avenues for potential therapeutic intervention could be opened. As Dr. Sarah Thompson, a contributing author of the study stated, “Our findings provide a new framework to consider how lipid profiles within mitochondria can be targeted to restore proper function in cells.”
In summary, this study provides a compelling view into the molecular interactions occurring within mitochondria, suggesting that specific lipids such as DAG are not mere bystanders but active players in managing cellular health. As research continues, understanding how varying lipid compositions affect mitochondrial dynamics may illuminate fresh pathways for therapeutic development tailored to combat diseases linked to mitochondrial dysregulation.