Scientists have discovered a new way to create biomolecular condensates using short peptides, revealing unique core-shell structures that could transform our understanding of molecular interactions in biological systems.
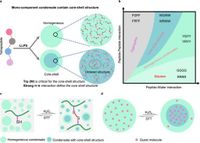
A team of researchers has reported the formation of unique core-shell structural biomolecular condensates through the liquid-liquid phase separation of tetrapeptides. These condensates, composed of just a single type of short peptide, offer insight into the underlying molecular mechanisms that govern the assembly and functionality of cellular compartments.
The driving force behind the formation of these condensates lies in the amino acid tryptophan, known for its robust π-π stacking interactions, which enable the creation of solid-like inner cores surrounded by more fluid outer shells. Using advanced techniques such as molecular dynamics simulation, cryogenic electron microscopy, and focused ion beam scanning electron microscopy, researchers characterized the structural properties of these condensates, marking a significant advancement in synthetic biomaterial design.
In essence, the formation process involves tuning the composition and environmental conditions, revealing that the inner cores of the condensates are organized, contrasting with the liquid-like characteristics of the outer layers. This hierarchical architecture is thought to enhance cellular processes, including signaling and material transport, by spatially organizing biomolecules within cells to facilitate efficiency.
Moreover, the researchers highlighted the potential applications of these findings, suggesting that such synthetic multiphasic condensates could be engineered to serve roles in drug delivery or as therapeutic vehicles, where precise control over phase separation and interaction dynamics may pave the way for new treatments.
In the laboratory, researchers manipulated the environment of these peptides, achieving dynamic control over the condensate formation through processes like redox reactions and post-translational modifications. The ability to program the assembly of such biomolecular structures offers new pathways in biochemical research and material science.
Core-shell structures can be finely tuned, with peptide composition impacting their stability and functionality at different pH and temperature conditions. The regeneration potential—transforming multiphasic condensates into homogeneous states under specific stimuli—opens exciting opportunities for practical applications in biomimetic systems, where programmable behavior is essential.
Dr. H. M. Wang, one of the leading researchers on this study, emphasized the importance of tryptophan's role in the structural integrity of the condensates. "The strongest interactions found in our study highlight the significance of specific amino acids in biomolecular architecture," stated the authors of the article.
This discovery underlines the evolving understanding of protein interactions and phase behavior in complex biological systems. As researchers continue to explore the potential of such synthetic condensates, their findings may contribute significantly to our grasp of cellular organization, ultimately informing the development of new technologies that mimic these natural phenomena.
Overall, the innovative use of tetrapeptides in forming structured biomolecular condensates showcases the intersection of biology and chemistry, paving the way for the rational design of materials tailored for specific applications in various scientific fields.