A recent study has illuminated a promising avenue in the battle against Parkinson's disease (PD), revealing that the probiotic bacterium Bacillus subtilis may confer significant neuroprotective effects. Parkinson's disease, characterized by the progressive loss of dopamine-producing neurons in the substantia nigra, affects millions worldwide. With no curative treatment currently available, research aimed at understanding protective strategies against neurodegeneration is more crucial than ever.
The study, led by researchers M. Francisco and R. Grau, investigated the interactions between B. subtilis and Caenorhabditis elegans, a worm model frequently used in neurodegenerative research. They discovered that B. subtilis, when used to colonize these worms, could prevent neuronal injury induced by the neurotoxin 6-hydroxydopamine (6-OHDA), a chemical compound associated with PD. Importantly, biofilm-forming strains of B. subtilis showed remarkable resistance to the neurotoxic effects that typically lead to neuron death and impaired functionality.
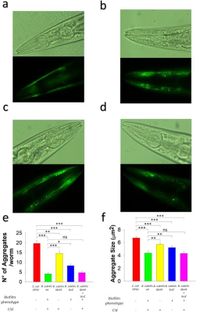
In their findings, the researchers noted that worms colonized by biofilm-forming B. subtilis displayed dopamine-dependent behaviors similar to those of untreated worms. This suggests that the probiotic not only prevents injury but also helps maintain normal neuronal function. The experiment also tracked the aggregation of human alpha-synuclein, a protein closely linked to PD. The results were remarkable: B. subtilis NCIB3610 diminished the quantity and size of alpha-synuclein aggregates by approximately 77% and 34%, respectively.
Additionally, the colonization of C. elegans with B. subtilis was shown to activate key signaling pathways involved in neuron health. Specifically, the probiotic triggered PMK-1 (p38 MAPK) and SKN-1 (Nrf2) signaling pathways, known for their roles in cellular stress responses and protection against neurotoxin-induced damage.
The protective benefits of B. subtilis were not limited to chemically induced damage. The researchers also noted that the probiotic could slow down age-related neurodegeneration in C. elegans. By using worms genetically modified to accelerate dopaminergic neuron decay, they verified that biofilm-forming strains of B. subtilis could prolong neuronal integrity and even enhance the lifespan of the worms.
Beyond these immediate findings, the implications for Parkinson's research are profound. With more than five million individuals currently affected by PD, and projections suggesting that numbers will double in the coming decade, identifying effective treatments is critical. This study adds a new layer to our understanding of how gut microbiota may influence neurological health. Biofilm-forming B. subtilis appears to address not only the symptoms but also some of the underlying mechanisms of neurodegeneration, aligning well with existing literature that highlights the connection between gut health and brain function.
The authors emphasize the potential of probiotics, particularly B. subtilis, in future dietary and therapeutic strategies for managing Parkinson's disease. This study opens the door for further exploration into how modulating gut microbiota can protect against neurological pathologies that have long resisted effective treatment.
In conclusion, the findings underscore the promise of probiotics like B. subtilis as neuroprotective agents in the quest to combat Parkinson's disease. As we continue to explore this innovative approach, the goal remains to transform how we understand and treat neurodegenerative diseases, possibly changing the course of treatment for millions around the globe.