In a breakthrough that could transform the way hydrogen fuel is produced, researchers have developed a novel support-free iridium catalyst for use in proton-exchange membrane water electrolyzers (PEMWEs). This innovative catalyst utilizes a unique metal-oxide-based molecular self-assembly strategy that allows for high performance in the oxygen evolution reaction (OER), a crucial component in sustainable hydrogen generation.
The self-assembled catalyst features densely isolated single IrO6H8 octahedra, resulting in the formation of hierarchically porous iridium hydroxide particles. These particles exhibit exceptional catalytic activity, registering a turnover frequency of 5.31 s⁻¹ at 1.52 V within the membrane-electrode assembly, while achieving a cell voltage of less than 1.75 V at a current density of 4 A cm⁻² with only 0.375 mg/cm² iridium loading.
Electrochemical technologies that harness renewable energy, such as water electrolysis, are essential for developing sustainable energy solutions. As the demand for clean hydrogen fuel escalates, the efficiency of these technologies becomes ever more crucial. Currently, the high kinetic barrier presented by the OER remains a significant challenge, often limiting the efficacy of conventional catalysts to iridium-based materials.
This study emphasizes the need for reducing iridium usage while maintaining superior performance amid harsh acidic conditions, where most non-precious metal OER catalysts struggle significantly. Past approaches utilizing state-of-the-art iridium catalysts like rutile IrO2 have required high loading to offset their low intrinsic activity, leading to concerns regarding cost and sustainability.
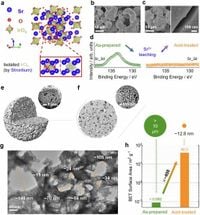
The approach outlined in this study introduces an advanced Sr4IrO6 precatalyst capable of minimizing iridium consumption while maintaining impressive performance. By employing an acid leaching process on Sr4IrO6, researchers successfully crafted an iridium catalyst with extraordinary turnover frequencies and cell voltages. The result is a catalyst that not only boosts the overall activity but also enhances mass transport due to its unique porous architecture.
The results show that the self-assembled hierarchically porous structure of the catalyst contributes to a remarkable approximately 400-fold increase in surface area compared to non-treated models. This high surface area paves the way for improved electrocatalytic activity and better access to active sites, essential for optimizing reactions in PEMWEs.
Tests conducted on the new HP-IrOxHy catalyst have indicated stable performance over prolonged hours of operation, with the S-number metric rising significantly from approximately 1.5 × 10⁸ to over 1.1 × 10⁹ during extended tests, validating its durability and effectiveness in practical applications.
Comparative evaluations, including other leading iridium-based catalysts, reveal that the innovative HP-IrOxHy demonstrates not only superior catalytic qualities but also operational stability, even under fluctuating power conditions typical of renewable energy sources.
The implications of this research extend well beyond mere performance metrics; they suggest a scalable approach to developing support-free catalysts that can be used in a variety of electrochemical applications. Future work will likely focus on expanding this self-assembly technique to other catalyst materials, further advancing the technologies necessary for realizing a hydrogen economy.
This pioneering catalyst could usher in significant advancements in hydrogen production technology, aligning with global goals of sustainability and environmental responsibility. Researchers assert that the metal-oxide-based molecular self-assembly strategy presents a promising pathway for the next generation of high-performance electrocatalysts.