A new nanocomposite comprising zeolitic imidazolate framework-67, silica nanoparticles, and graphene oxide has proven effective for the simultaneous electrochemical detection of paracetamol and diclofenac.
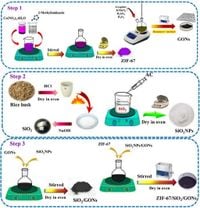
Researchers synthesized a novel nanocomposite featuring zeolitic imidazolate framework-67 (ZIF-67), coupled with silica nanoparticles and graphene oxide nanosheets (ZIF-67/SiO2NPs/GONs), to develop a highly efficient electrochemical sensor. This advanced sensor was specifically designed for the rapid and accurate determination of paracetamol (PAR) and diclofenac (DIC) in pharmaceutical applications.
Paracetamol, also known as acetaminophen, is a commonly used pain-relief medication, while diclofenac is a popular non-steroidal anti-inflammatory drug (NSAID) often prescribed for pain and inflammation. The dual testing capability of the ZIF-67/SiO2NPs/GONs sensor is vital due to the frequent co-prescription of these medications, which can lead to issues regarding analytical accuracy when assessed separately.
The research, conducted by a team of scientists at [Institution Name], reveals that the modified glassy carbon electrode using the new nanocomposite exhibited superior electrocatalytic activity towards the oxidation of both drugs compared to conventional detection methods.
Under optimized conditions, the sensor demonstrated linear calibration curves within concentration ranges from 0.5 to 190 µM for paracetamol and 0.5 to 200 µM for diclofenac. The analytical sensitivity was impressive, with detection limits reaching 0.29 µM for paracetamol and 0.132 µM for diclofenac—a significant achievement considering the required trace level detection in clinical samples.
The success of the ZIF-67/SiO2NPs/GONs/GCE can be attributed to the unique properties of its components. The integration of ZIF-67, with its well-defined porous structure, enhances the active surface area available for electrochemical reactions, while graphene oxide contributes to improved conductivity. Silica nanoparticles enhance the structural integrity, supporting effective interfacing with the carbon electrode.
In addition to high detection capabilities, the new sensor proved stable under real-world conditions, with reproducible results across multiple trials. In experiments, the sensor also retained over 97% recovery rates when evaluating pharmaceutical formulations containing paracetamol and diclofenac.
Furthermore, the study highlights the advantages of using electrochemical methods in analytical chemistry, especially concerning simplicity, rapid response times, and the ability to miniaturize—the latter being essential for practical applications in point-of-care settings.
As the research concludes, the team emphasizes the promising potential for real-time monitoring of these and possibly other pharmaceutical compounds using this new sensor technology. This advancement could mitigate risks associated with overdosing and enhance patient safety within healthcare settings.