A groundbreaking study has unveiled a patterned homeostatic human gastric organoid-on-a-chip model aimed at deepening the understanding of Helicobacter pylori (H. pylori) infections, which are linked to various gastric diseases including cancer and peptic ulcers.
The research, published in March 2025, highlights the persistent challenges that H. pylori poses, as it can colonize the gastric epithelium and inflict cellular damage over time, often leading to serious health complications. Traditional models for studying these infections have been inadequate; commonly used two-dimensional systems fail to mimic the stomach's structural complexity and three-dimensional systems are often too complex for effective experimental access.
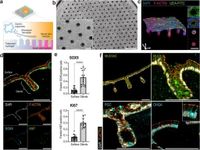
The newly developed organ-on-a-chip system allows for bilateral access to the gastric epithelium, facilitating a more accurate replication of physiological conditions, including acidic environments similar to those found in the human stomach. This model improves the generation of mature gastric pit cells, which exhibit distinct responses to H. pylori infections, setting it apart from traditional organoid cultures.
Under physiological apical acidic conditions, researchers were able to achieve higher maturity levels of pit cells within the organ-on-a-chip compared to those grown conventionally. This is significant; mature pit cells respond differently to H. pylori, offering insights into their pivotal role in chronic infection dynamics.
"In physiological apical acidic conditions, our organ-on-a-chip can generate pit cells of higher maturity in contrast to traditionally grown organoids," wrote the authors of the article, affirming the model's utility in studying the early stages of H. pylori colonization.
The study utilized single-cell RNA sequencing to analyze cell responses to infection, revealing that all cell types within the gastric epithelium reacted to H. pylori, albeit in notably distinct ways. Progenitor and immature pit cells predominantly exhibited an inflammatory response, while mature pit cells showed a unique upregulation of genes related to cellular junction integrity and antibacterial responses.
Importantly, the research observed the bacteria's ability to create a microenvironment that protects against acidity. Confocal imaging experiments confirmed this attachment to cell-cell junctions, indicating that H. pylori can maintain its presence within acidic conditions without severely damaging the epithelial monolayer. "The bacterial burden remained stable over time, a key feature for modeling chronic infection," stated the authors, underscoring the implications for long-term studies of H. pylori.
The findings also highlighted that mature pit cells in acidic environments dramatically increased the expression of dual oxidase proteins, crucial for antimicrobial processes, further demonstrating their role in gastric health. The researchers noted, "We identified additional responses specific to mature pit cells that had not been observed before," expanding the understanding of how these cells influence infection outcomes.
Overall, the organoid-on-a-chip model presents a promising platform for enhanced exploration of gastric epithelial interactions with H. pylori under biologically relevant conditions. The ability to simulate long-term infections opens new avenues for research aimed at elucidating the progression of gastric diseases associated with this persistent pathogen.
As researchers continue to explore the complexities of H. pylori infections, this homeostatic model not only sheds light on host-pathogen dynamics but also holds potential for studying other gastric health challenges in the future. This work emphasizes the importance of innovative approaches in microbiome research and offers a significant step forward in understanding gastric health and disease.