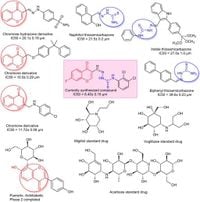
In a significant advancement for diabetes treatment, researchers have synthesized a new series of chromone derivatives featuring thiosemicarbazones that show potent a-glucosidase inhibition, offering potential new therapies for Type 2 Diabetes Mellitus (T2DM).
Type 2 Diabetes Mellitus is a major health concern worldwide, affecting over 536 million people as of 2021, and is projected to affect nearly 783 million by 2045. This form of diabetes is characterized by insulin resistance and elevated blood sugar levels, exacerbated by the enzymatic breakdown of carbohydrates in the gut. The enzyme a-glucosidase plays a central role in this process, leading to increased postprandial blood glucose levels.
To combat this, researchers have explored innovative a-glucosidase inhibitors, specifically thiosemicarbazones combined with chromones, a class of natural compounds known for various therapeutic properties. The study synthesized 20 new compounds, which were tested for their ability to suppress a-glucosidase activity. The research found that a compound labeled 3k exhibited the highest inhibitory action with an IC50 value of 6.40 ± 0.15 μM, dramatically outpacing the benchmark drug acarbose, which had an IC50 value of 870.36 ± 1.24 μM.
The synthesis of these compounds involved reacting 7-fluoro-4-oxo-4H-chromene-3-carbaldehyde with various thiosemicarbazides to yield stable thiosemicarbazone derivatives. The authors determined that substituents on the thiosemicarbazone moieties significantly influenced their a-glucosidase inhibition potential.
"The structural characteristics of these chromone-thiosemicarbazone hybrids indicate promising activity against a-glucosidase, potentially leading to their further development as therapeutic agents for managing T2DM," noted the authors of the article.
In addition to the experimental work, the researchers utilized molecular docking studies to examine the binding interactions between the most potent compound and the a-glucosidase enzyme. Key interactions included π-π bonds and hydrogen bridges, which were critical for the stability and effectiveness of the enzyme-inhibitor complexes.
The study concluded that these chromone-thiosemicarbazone hybrids represent a new class of a-glucosidase inhibitors with enhanced therapeutic potential for diabetes management. Furthermore, the findings highlighted the necessity for continued optimization and pharmacokinetic profiling of these compounds to ensure their efficacy and safety in clinical settings.
This innovative approach not only addresses a pressing healthcare need but also paves the way for future research into the development of new diabetes medications that could alleviate the burden associated with diabetes complications.
As the prevalence of diabetes continues to rise globally, the identification of novel and more effective therapeutic options is paramount. This research could be a turning point in developing next-generation a-glucosidase inhibitors that enhance glycemic control while minimizing side effects traditionally associated with current therapies.
The findings underscore the importance of interdisciplinary research approaches in drug discovery, combining elements of medicinal chemistry, pharmacology, and computational biology to yield effective new treatments.