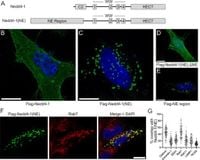
A newly identified isoform of the ubiquitin ligase Nedd4-1, termed Nedd4-1(NE), shows distinct properties that could reshape our understanding of cellular regulation in primates. This research focuses on how Nedd4-1(NE) diverges from its canonical counterpart, which is primarily cytosolic, instead localizing specifically to late endosomes. This localization is not arbitrary; it shifts based on amino acid levels and is influenced by the activity of the protein IKKβ, providing a nuanced mechanism of cellular adaptation in response to nutrient availability.
While traditional roles of ubiquitin ligases like Nedd4-1 involve tagging proteins for degradation, Nedd4-1(NE) appears to adopt a unique function, particularly in the context of autophagy—a critical process for cellular maintenance that involves the degradation of dysfunctional components through lysosomal pathways. By interacting with VPS16B, which plays a significant role in endosomal maturation, Nedd4-1(NE) is implicated in regulating when and how these cellular processes occur.
The study's findings are particularly significant given the discovery of a pathogenic variant (E70Q) associated with a patient suffering from lymphangiectasia, a condition characterized by protein loss in the intestines. This variant reduces the ability of Nedd4-1(NE) to ubiquitinate VPS16B, highlighting how genetic alterations can impact fundamental cellular operations, leading to disease.
Importantly, the researchers established that Nedd4-1(NE) localization to late endosomes occurs as a response to the availability of amino acids, operating independently of the mTORC1 signaling pathway—a conventional regulator often tied to nutrient sensing and cellular growth. Instead, the findings suggest an alternative regulatory mechanism influenced by IKKβ, which is known for its role in promoting autophagy under nutrient deprivation scenarios.
Experimental approaches included immunofluorescence microscopy to visualize the spatial distribution of Nedd4-1(NE) within cells, distinguishing its endosomal presence from the canonical Nedd4-1 localization pattern. Moreover, the study leveraged proteomic tools to unearth the binding partners of Nedd4-1(NE), revealing VPS16B as a critical substrate involved in modulating the autophagic process.
These revelations about Nedd4-1(NE) not only enhance our fundamental understanding of ubiquitination processes in primates but also raise important questions regarding the potential implications for therapeutic strategies targeting related diseases. Understanding how this isoform operates could pave the way for innovative approaches to tackle conditions linked to autophagy dysfunctions.
In conclusion, the study underscores the intricate relationship between nutrient availability and ubiquitin ligase function in primates, suggesting that Nedd4-1(NE) serves as a unique model for exploring species-specific adaptations in cellular regulation. Exploring this pathway further may yield significant insights that inform future research and clinical strategies to address analogous human health issues.