In a significant advance for stem cell research and drug testing, new techniques have been developed to enhance the maturation of induced pluripotent stem cell-derived cardiomyocytes (iPSC-CMs). Researchers have systematically combined lipid-enriched maturation mediums, elevated calcium concentrations, nanopatterned surface designs, and electrostimulation to produce iPSC-CMs that exhibit more adult-like characteristics, paving the way for better pharmacological assessments.
iPSC technology, which allows for the generation of cardiomyocytes from a patient's own cells, holds promise for regenerative medicine and precision therapies. However, the immaturity of these cells has posed significant challenges in evaluating the effects of drugs, especially the risk of arrhythmias and cardiotoxicity. Adult cardiomyocytes present a more accurate model for testing, emphasizing the need for methods that can push iPSC-CMs closer to their mature counterparts.
In their study, researchers identified that electrostimulation was the key factor driving advancements in mitochondrial development and metabolic maturation of iPSC-CMs. "Systematic testing reveals that electrostimulation is the key driver of enhanced mitochondrial development and metabolic maturation and improved electrophysiological properties of iPSC-CMs," stated the authors of the article.
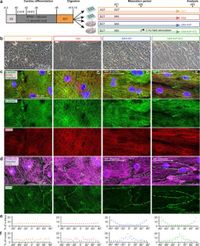
There are several maturation techniques for iPSC-CMs, including the addition of fatty acids and hormones, and the use of engineered heart tissues. New findings showed that increasing the calcium concentration in the culture medium significantly improved the electrophysiological properties of iPSC-CMs. Notably, the nanopatterning of culture surfaces mainly aided in structural maturation, demonstrating that a multifaceted approach is most effective.
Since adult CMs significantly differ from iPSC-CMs in terms of morphology, gene expression, metabolism, and functionality, these advancements highlight pathways that improve their resemblance to mature cells. The researchers comprehensively analyzed the changes in the cells and the outcomes of their methods using functional and molecular analyses.
After systematically applying the lipid-enriched maturation medium combined with a high concentration of calcium, nanopatterning, and electrostimulation, significant improvements were reflected in the structural and electrophysiological characteristics of the iPSCs. The study detailed observations showing organized sarcomeres and better cell alignment in enhanced conditions compared to conventional techniques.
More specifically, the results revealed that up to 43% of iPSC-CMs in the most advanced conditions showed a ‘notch-and-dome’ action potential morphology, a feature absent in less mature groups. Additionally, resting membrane potentials became more negative, and other critical electrophysiological parameters improved dramatically.
As a direct consequence of these changes, the pharmacological response of mature iPSC-CMs displayed remarkable enhancements. The authors wrote, "These findings provide mechanistic insights into iPSC-CM maturation, paving the way for pharmacological responses that more closely resemble those of adult CMs.” This offers exciting prospects for their utilization in drug screening and deeper understanding of heart disease mechanisms.
Furthermore, the study notes significant downregulation in pathways that supported cell division, indicating that the increased maturity of iPSC-CMs leads to polyploidy, a common feature in adult hearts. Concurrently, molecular analyses suggested that metabolic and structural maturation is tied to exercise on calcium handling and mitochondrial function, reinforcing the increasing complexity of these cell types.
As the research continues to chart a course through the intricacies of stem cell-derived cardiomyocytes, these findings underscore the driving forces behind their maturation and initial steps toward their viability in real-world medical applications. Enhancing maturity may ultimately aid biomedical advances, enabling personalized therapies for heart disease.
While additional studies will be needed to analyze how these enhancements influence long-term cell behavior and their potential for clinical translation, the present findings signify a key development in addressing both pharmacological safety and regenerative capabilities. As we move forward, the hope for patient-specific therapies grows, driven by the potential of improved iPSC-CMs.