Research on dopamine's effect on working memory has revealed intriguing insights, particularly about the dopamine D1 receptor. A recent study investigates the dual nature of D1 receptor stimulation, showcasing how it can both impair and expand working memory capacity (WMC) depending on the dose administered.
Working memory, the brain’s ability to hold and manipulate information for short periods, is not just about duration; it has capacity limits. Generally, humans can manage around six items at once, but this ability is significantly reduced under various conditions, such as schizophrenia and aging. Indeed, reduced WMC is a core cognitive impairment observed in schizophrenia patients, emphasizing the importance of this research.
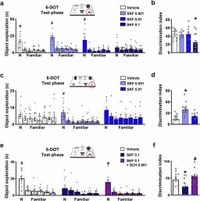
The study examined the effects of D1 receptor stimulation, using the selective D1 agonist SKF 38393 on mice. It was discovered through behavioral tests, including the different/identical objects test, which evaluated the mice’s memory capacity under varying object loads.
Low doses of SKF 38393, at 0.001 mg/kg, led to notable improvements. Mice administered this dose were able to successfully distinguish between eight different objects, effectively enhancing their WMC beyond typical physiological limits. "Low doses of the D1 agonist SKF 38393 expand incidental WMC in mice and rescue WMC deficits in an animal model of schizophrenia," stated the authors of the article.
Conversely, at higher doses, observed at 0.1 mg/kg, there was significant impairment. This decline was linked to elevated activity within the medial prefrontal cortex (mPFC) and the recruitment of inhibitory striatal parvalbumin interneurons. This phenomenon can be attributed to the 'inverted-U shaped' dose-response curve characteristic of dopamine’s cognitive effects. Here, both insufficient and excessive stimulation can derail memory performance, rendering the appropriate dose of dopamine pivotal for cognitive enhancement.
The underlying mechanisms were carefully examined. The activation of the cAMP/PKA signaling pathway was central to this study. When low doses were administered, the cAMP/PKA pathway was activated within the striatum, enhancing WMC. On the other hand, higher doses activated cAMP/PKA pathways within the mPFC but simultaneously inhibited them within the striatum. This duality leads to altered behavior across memory tasks, providing insight on the complex interactions between these brain regions.
To investigate this more thoroughly, researchers utilized several advanced techniques—pharmacology, optogenetics, and chemogenetic approaches—revealing substantial interactions. Using optogenetic inhibition of the mPFC-striatal pathway prevented memory impairments induced by high doses of SKF 38393, allowing researchers to validate their findings on how exact signaling can switch between facilitating or impeding memory processing.
Through these experiments, the research team underscored the importance of taking a comprehensive systems pharmacology approach, which considers how interconnected brain systems may influence cognitive functions rather than just the isolated effects of drug-receptor interactions.
Importantly, the findings have tremendous relevance for conditions like schizophrenia, where cognitive deficits contribute substantially to the disorder’s burden. By demonstrating how low doses of D1 receptor stimulation can restore WMC deficits—"These results highlight the need for systems pharmacology to develop memory-enhancing treatments,” emphasized the researchers.
The results are promising, especially since traditional antipsychotic treatments do little to alleviate cognitive symptoms associated with schizophrenia. Contrarily, the administration of SKF 38393 significantly improved memory loss observed following MK-801-induced deficits, providing hope for addressing cognitive symptoms effectively.
This work adds depth to existing knowledge, prompting questions about how these findings can be translated to human therapies. There remains much to elucidate about the mechanisms underlying these complex interactions, but the study paves the way for targeted treatment approaches aimed at bolstering working memory capacity, particularly for those struggling with cognitive impairments caused by psychiatric conditions.