Aging is a ubiquitous process that affects organisms across the spectrum of life, leading to a gradual decline in cellular function and an accumulation of senescent cells in various tissues. Recent research has highlighted the intricate relationship between aging, gene expression, and chromatin accessibility, with a specific focus on the role of the transcription factor FOXM1.
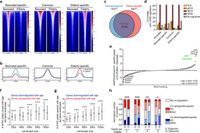
According to a study examining chromatin accessibility in human dermal fibroblasts (HDFs) across a range of ages from neonates to octogenarians, age-related changes in chromatin structure significantly influence gene expression and play a pivotal role in promoting senescence. The researchers employed a technique called Assay for Transposase-Accessible Chromatin using sequencing (ATAC-seq) to reveal how the regulatory landscape around key genes transforms with age.
The study found that as fibroblasts age, the accessibility of chromatin changes dramatically; regions enriched with AP-1 binding motifs were more prevalent in elderly fibroblasts, whereas neonate-specific regions were rich in TEAD binding motifs. This shift indicates that the dynamics of transcription factors (TFs) change significantly with age, reflecting their influence on cellular aging and senescence.
One key finding of this research was the discovery of an enhancer region, termed C10, which exhibits age-dependent accessibility. This region is crucial for regulating the expression of FOXM1 and other related genes. Deletion of the C10 enhancer led to decreased expression levels of FOXM1, TEAD4, and RHNO1—genes known to be involved in cell proliferation, DNA damage repair, and maintaining cellular health. This loss of expression resulted in increased senescence markers, underscoring the enhancer's essential role in promoting cellular youthfulness.
"We demonstrate that FOXM1 ectopic expression in elderly cells partially resets chromatin accessibility to a youthful state due to FOXM1’s repressive function on several members of the AP-1 complex," wrote the authors of the article. The repressive action of FOXM1 suggests that during aging, its downregulation may lead to a more open chromatin state, thus enabling promoters of senescence-associated genes to become active, which in turn contributes to cellular aging.
The researchers also highlighted that changes in chromatin accessibility correlate with transcriptional shifts in genomic landscapes. Genes whose expression is upregulated during aging exhibited increased elderly-specific open chromatin peaks, suggesting that regulatory elements respond to age-related cellular states. Conversely, the number of neonatal-specific peaks was found to be more prevalent in genomic areas of downregulated genes, indicating a decrease in youthful transcriptional control with advancing age.
The study further validated the functional importance of the C10 enhancer through CRISPR/Cas9-mediated deletions, revealing that losing this enhancer triggers senescence phenotypes even in younger HDFs. Such results provide compelling evidence linking chromatin remodeling and transcriptional alterations to the aging process.
In addition, the research posits that FOXM1 overexpression in elderly fibroblasts can restore a more youthful chromatin profile. Ectopic expression of FOXM1 resulted in a partial closure of chromatin regions that would typically remain open in elderly cells, which affirms its role as a repressor of the senescence transcriptional program. This provides significant implications for potential therapeutic strategies aimed at reversing age-associated functional declines by targeting regulatory elements and transcription factors.
Ultimately, the study reveals that aging induces profound changes in chromatin accessibility that are tightly linked to changes in gene expression and cellular function. With genes like FOXM1 appearing to play a crucial role in regulating age-associated transcriptional programs, there are exciting avenues for further research and potential interventions aimed at mitigating the effects of aging.