In a groundbreaking study published recently, scientists have unveiled critical insights into the role of 5-hydroxymethylcytosine (5hmC), often referred to as the sixth base of DNA, in relation to Alzheimer’s disease (AD). This investigation provides a comprehensive genome-wide mapping of 5hmC throughout the brains of over one thousand older individuals, revealing approximately 3,000 differentially hydroxymethylated regions (DhMRs) that are closely associated with the neuropathological features characteristic of Alzheimer’s.
Alzheimer’s disease is a devastating neurodegenerative disorder that impacts over 35 million individuals globally. The illness is characterized by a variety of pathological hallmarks, including the buildup of extracellular amyloid plaques and the formation of neurofibrillary tangles, which progressively lead to neuronal loss. Although significant attention has been given to genetic factors influencing AD, new research highlights the growing evidence supporting the role of epigenetic modifications in exacerbating or mitigating disease progression.
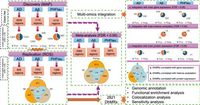
The study, involving researchers from various leading institutions, primarily focused on the dorsolateral prefrontal cortex (DLPFC) of participants from the Religious Orders Study and the Rush Memory and Aging Project. By employing a technique known as selective chemical labeling sequencing, or hMe-Seal, researchers mapped the distribution of 5hmC across the brains of 1,079 deceased individuals, all of whom were evaluated for cognitive impairment prior to their death. Notably, the average age of participants was 89 years, encompassing a diverse representation of older adults.
Once the 5hmC profiles were established, the team set out to identify associations between these epigenetic alterations and a range of Alzheimer’s pathology features, funding their research with extensive clinical data and brain donation records. The results from the study were striking; the authors reported that their genome-wide atlas revealed 2,821 DhMRs associated with Alzheimer’s neuropathology after accounting for several covariates. As the authors of the article stated, "Our study presents a large-scale genome-wide atlas of 5-hydroxymethylcytosine in Alzheimer’s brain and offers insight into the mechanism underlying Alzheimer’s disease pathogenesis.”
A significant aspect of the findings was the revelation that higher levels of 5hmC corresponded with a lower risk of Alzheimer’s disease diagnosis and lower counts of amyloid plaques and tau tangles. Conversely, specific regions exhibited elevated 5hmC levels linked to increased risks of AD pathology. The extensive data analysis also revealed that 93 of the identified DhMRs were significantly associated with all three pathological features of Alzheimer’s: diagnosis, amyloid-β load, and tau tangles.
Moreover, integrative multi-omics analyses conducted within the study bolstered the notion that changes to 5hmC may impact the activity of nearby genes and thus contribute to AD. As captured in the findings, "Integrative multi-omics analyses support a potential mechanistic role of 5-hydroxymethylcytosine alterations in Alzheimer’s disease". This indicates that alterations in DNA hydroxymethylation could be crucial for understanding how certain genetic and environmental factors interact to influence Alzheimer’s pathology.
The research also identified numerous DhMRs located within previously understood AD loci, including genes such as RIN3, PLCG2, ITGA2B, and USP6NL. This suggests that the mechanism by which 5hmC influences AD could be mediated by pathways previously erased by traditional genetic studies. Providing new pathways for therapeutic exploration, these results underline the importance of considering epigenetic marks in the context of neurodegeneration and may lead to more effective treatment options for those at risk of or affected by Alzheimer’s disease.
As this research lays the groundwork for further exploration, it suggests that future studies could validate the role of 5hmC as a potential biomarker for Alzheimer’s disease risk. The loss of DNA hydroxymethylation was noted to be significantly higher among those diagnosed with AD, indicating that it may serve as a critical indicator of disease progression.
In conclusion, this extensive analysis of 5hmC significantly enhances our understanding of the epigenetic factors that contribute to Alzheimer’s disease pathology. The discovery of 2,821 DhMRs associated with key pathological features paves the way for future research to isolate and explore these associations further, which could eventually lead to novel therapeutic interventions aimed at preserving cognitive function and developing effective strategies for combating Alzheimer’s disease.