In a groundbreaking study that could revolutionize treatment options for triple-negative breast cancer (TNBC), researchers have identified a promising new molecular target: the somatostatin receptor 2 (SSTR2). This receptor, which is often overexpressed in various cancers, could be a key player in providing targeted therapy for patients whose tumors lack common therapeutic targets such as the estrogen receptor and human epidermal growth factor receptor 2 (HER2).
Recent findings published by a team of researchers from the University of Alabama at Birmingham in Scientific Reports demonstrate that SSTR2 expression can be significantly enhanced through the use of histone deacetylase (HDAC) inhibitors. This approach not only elevates the protein’s levels but also makes it possible to track changes in SSTR2 expression using positron emission tomography (PET) imaging.
Breast cancer remains one of the leading causes of cancer-related mortality among women across the globe, with TNBC being one of its most aggressive forms. In TNBC, the absence of molecular targets can severely limit a patient’s treatment options. Current therapies primarily focus on chemotherapy, which do not work effectively for all patients. Hence, discovering novel biomarkers like SSTR2 is essential for advancing treatment, as noted by the authors of the article.
“Due to the lack of therapeutic targets, there are currently no approved targeted therapies for patients with TNBC,” they commented, highlighting the urgent need for reliable and targetable biomarkers. Their research delves into the use of known HDAC inhibitors, including suberoylanilide hydroxamic acid (SAHA), to stimulate SSTR2 expression in preclinical models.
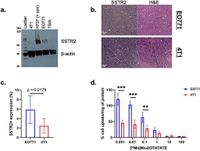
The researchers utilized various mouse mammary carcinoma cell lines to assess SSTR2 levels, discovering significant variability in expression across models. For example, mouse-derived EO771 cells exhibited relatively high SSTR2 expression compared to 4T1 cells, which showed lower levels. Importantly, after treatment with SAHA, researchers observed a remarkable increase in SSTR2 expression—up to four times for EO771 and up to eight times for 4T1 cells.
The findings were emphasized in a series of non-invasive tests using PET imaging with the tracer [68Ga]Ga-DOTATATE, which binds to SSTR2. This imaging technique demonstrated a clear ability to visualize SSTR2 expression dynamically, allowing for longitudinal monitoring of cellular changes post-treatment with SAHA.
“SSTR2 expression can be characterized non-invasively via PET imaging,” the authors stated, confirming the method’s usefulness in longitudinal studies.
Further demonstrating the impact of HDAC inhibitors, the study revealed that SAHA administration not only amplified SSTR2 at the transcriptional and translational levels but also enhanced the receptor’s functional capabilities. The data gathered displayed significant increases in cellular uptake of marketed somatostatin analogues, correlating directly with elevated SSTR2 levels. In vitro tests showed an increase of up to 200% in DOTATATE uptake in EO771 cells following SAHA treatment, a promising sign for potential therapeutic applications.
In vivo studies reinforced these findings. The mouse models treated with SAHA showed improved overall survival rates, achieving statistical significance as compared to untreated control groups. Specifically, EO771 tumor-bearing mice exhibited a survival increase of 36% when treated with the HDAC inhibitor.
“Treatment with SAHA significantly increased overall survival for both EO771 and 4T1 tumor-bearing mice compared to control groups,” the authors noted, suggesting this therapy could offer newfound hope for those affected by this hard-to-treat subtype of breast cancer.
The implications of this research extend beyond mere technical advancements; the ability to non-invasively evaluate SSTR2 expression opens the door to increased personalized treatment planning in clinical settings. The potential to combine effective imaging techniques with targeted therapies could vastly improve patient management.
“Our findings suggest that epigenetic modulation of SSTR2 using HDAC inhibitors is a viable targeted strategy for imaging and therapy in breast cancers which have low or variable levels of SSTR2,” the authors concluded, reaffirming their confidence in the translational potential of their work.
As researchers continue to explore the intricate relationships between gene expression and cancer therapies, the study of SSTR2 serves as a promising avenue toward a future with more effective treatment regimes for those diagnosed with TNBC. The integration of specialized imaging techniques alongside innovative therapies may enable more patients to benefit from targeted treatment options that were previously out of reach, changing the paradigm of care in breast cancer treatment.