Cryo-electron microscopy has unveiled distinct structural forms of aquaglyceroporins, specifically focusing on rat aquaglyceroporin AQP3 and its bacterial counterpart, GlpF. These proteins, known for facilitating the transport of water and small solutes, exhibit remarkable structural diversity
.Aquaglyceroporins such as aquaporin-3 (AQP3) and the bacterial GlpF are integral membrane proteins that ease the passage of water and glycerol across cellular membranes. They play critical roles in various physiological processes. Despite their importance, the structural characteristics governing their distinct functions, especially for AQP3, have remained poorly understood.
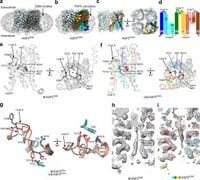
This study utilized advanced cryo-electron microscopy (cryo-EM) to elucidate the structures of AQP3 in different lipid environments, highlighting a previously unobserved conformation characterized by the insertion of an aromatic residue, Tyr212, into its pore. This conformational shift reflects a potential regulatory mechanism of permeability that distinguishes AQP3 from other aquaglyceroporins.
In the examination of AQP3's conformation, researchers noted two distinct states: one featuring a 2.8 Å diameter pore and another where Tyr212 is inserted, effectively narrowing the pore. Molecular dynamics simulations supported this finding, revealing the permeabilizing behavior changes associated with Tyr212's position. The authors of the article noted, "The narrowed pore structure of AQP3POPC through the extracellular loop E with the insertion of Tyr212 into the pore is an AQP conformation not previously observed." This unique characteristic indicates how the protein can regulate its permeability for solutes.
Similarly, the study explored GlpF, unveiling structural features where the pore is occluded by an intracellular loop. This flexibility of the loop allows for potential regulation of solute permeation, though it appears to play a lesser role in GlpF’s permeability compared to AQP3. These revelations contribute to a growing understanding of aquaglyceroporins and their varied functions in different biological contexts.
Both AQP3 and GlpF are positioned as vital components in cellular pathways involving osmotic balance and nutrient transport. By harnessing cryo-EM, the researchers provided critical insights into how specific residues drive their structural dynamics. "Our findings illuminate the unique AQP3 conformation and structural diversity of aquaglyceroporins," stated the authors, emphasizing the broader implications these discoveries hold for understanding channel regulation.
Ultimately, the insights gained from this study not only expand the knowledge about the structural biology of aquaglyceroporins but may also pave the way for developing specific inhibitors or enhancers that can modulate these channels in physiological and pathological processes.
The structural variations observed in AQP3 and GlpF further indicate a need for a reevaluation of the functional mechanics of aquaglyceroporins across species, informing future research into their roles in health and disease. As these proteins continue to draw attention, ongoing studies may lead to innovative applications in biotechnology and therapeutics, targeting aquaglyceroporins in diseases linked to fluid regulation and homeostasis.