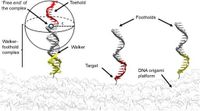
Scientists are unlocking the potential of DNA walkers, dynamic nanoscale structures that exhibit promising capabilities in molecular transport and mechanistic operations, by implementing innovative strategies to enhance their speed. Recent research published on March 19, 2025, explores how the principles of spatial confinement can significantly expedite the motion of these nanoscale machines, which are fundamental in various applications such as biosensing, nano-robotics, and molecular computing.
In the study, the authors investigate two primary strategies to enhance DNA walker efficiency: the introduction of tailed DNA footholds and the construction of trench-like geometries along the DNA track. The DNA walker system is designed to move through a series of binding sites, and traditionally, they face speed limitations predominantly stemming from the reaction-limited nature of strand exchange mechanisms.
By modifying the foothold structures to include a double-stranded DNA tail, the researchers have observed an impressive fourfold increase in the speed of DNA walkers. This technique promotes pseudo-rotational dynamics, allowing for smoother transitions between binding sites. The study demonstrated that these tailed footholds create more efficient sampling of binding configurations, substantially lowering the time it takes for a walker to find and attach to its next foothold. As stated by the authors of the article, "By focusing on the properties of the DNA track, this study offers novel insights into leveraging soft structural motifs to optimize signal propagation rates, with implications for sensing, robotics, and molecular computing in reaction-diffusion systems."
In addition to tailed footholds, the research assessed the implementation of trench-like geometries that guide walker motion more effectively. While this method yielded a more modest threefold increase in speed, it does require substantial structural modifications to the DNA track, which raises cost efficiency concerns. The trench confines the walker’s motion laterally while permitting forward movement, which also constrains the dynamics and reduces the entropy of the system.
The researchers found that by integrating both tailed footholds and trench configurations, the walker-foothold system exhibited bistable dynamics, wherein the walker could stabilize in one of two conformational states separated by an energy barrier. This dual-confinement approach not only enhances communication speed but also transforms the system into a more controllable mechanism for molecular transport. The authors comment that "the combination of tailed footholds and trench-like confinement turns the walker-foothold system bistable, with two distinct stable states separated by an energy barrier. This is the smallest synthetic DNA nanostructure which exhibits such bistable mechanism." Such bistable properties could pave the way for programming these nanoscale systems for targeted applications, such as biosensing platforms where the walker’s pathway changes in response to specific stimuli.
Using simulations to study these dynamics, the research team ran approximately 2 microseconds of simulated dynamics to achieve their results, demonstrating a significant leap in understanding how to enhance the operational capabilities of DNA walkers. The integration of more robust and precise control mechanisms could lead to stronger and more efficient designs in molecular robotics and sensing technologies.
This latest study exemplifies the rapid advancements being made in DNA nanotechnology and underscores the importance of structural manipulation to optimize molecular transport systems. The findings not only advance fundamental science but also open new pathways for practical applications in biotechnology, medicine, and materials science. As the field of dynamic DNA nanotechnology continues evolving, researchers are optimistic about the implications of these enhancements for the development of next-generation biosensing technologies and drug delivery systems, where speed and efficiency are paramount.