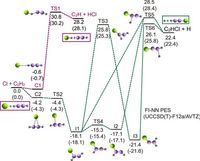
In a groundbreaking study, researchers have revealed that the chemistry of the reaction between chlorine and acetylene (C2H2) is far more complex than previously understood. Traditionally, it was believed that such bimolecular reactions proceeded through well-defined transition states. However, new insights indicate that the Cl + C2H2 reaction primarily unfolds through two nontraditional pathways known as roaming mechanisms.
Published on March 19, 2025, the research demonstrates that nearly 100% of the chemical yield from the Cl + C2H2 reaction arises from these roaming dynamics, specifically termed Cl-roaming and H-roaming. These findings significantly challenge long-standing theories in the field of chemical reactivity and dynamics.
"These findings challenge the traditional view of bimolecular reactions, emphasizing the importance of considering nontraditional pathways in reaction dynamics studies," wrote the authors of the article. Their work utilized full-dimensional dynamics simulations based on a sophisticated machine-learning-based potential energy surface, allowing for precise analysis of the reaction pathways.
The conventional approach to studying chemical reactions involved predicting outcomes through the minimum energy path (MEP), which typically focuses on transition states that dictate how bonds break and form during a reaction. However, roaming pathways present a departure from this framework, offering a more nuanced understanding of how molecules interact during reactions.
The roaming mechanisms observed in this study involve the temporary formation of intermediates that later react through unconventional routes. For instance, in the Cl-roaming mechanism, a transient C2H2Cl adduct forms first, allowing the chlorine atom to roam around and ultimately abstract a hydrogen atom from acetylene. Conversely, in H-roaming, a hydrogen atom detached from the molecule migrates and abstracts a chlorine atom instead.
This revelation is crucial as roaming pathways are fundamentally different from the expected direct abstraction pathway typically characterized by stable transition states. The authors emphasized that understanding these alternative mechanisms could significantly affect the predictions of reaction rates and outcomes.
The research findings highlight that at collision energies between 30 kcal/mol to 60 kcal/mol, Cl-roaming becomes the dominant pathway, contributing approximately 97% of the product yield at peak collision energy. The effective energies and angular distributions of the products were meticulously calculated, providing robust evidence for these claims.
"The dominance of the roaming mechanism over the conventional transition state mechanism is an important supplement to existing reaction rate theory," the authors stated. This point underscores the growing need to rethink fundamental models of chemical kinetics and dynamics.
The study's insights stem from a new machine-learning-based potential energy surface constructed from approximately 70,000 high-level quantum mechanical energy points, demonstrating a root-mean-square error of merely 11.39 meV across a broad energy spectrum. This level of accuracy is vital for capturing the complex interplay of reaction dynamics.
As researchers continue to investigate the implications of these findings, it opens up new avenues for future studies, particularly regarding how these roaming mechanisms may influence other chemical reactions involving heavy atoms like chlorine.
Furthermore, exploring the implications of these nontraditional pathways could yield unforeseen insights into atmospheric chemistry, where such reactions play a critical role. The understanding of Cl + C2H2 serves as an exemplar of the broader trends challenging our understanding of molecular interactions.
Thus, as scientific inquiry pushes deeper into the intricacies of chemical reactions, findings like those from this study emphasize the importance of adaptive methodologies and the incorporation of advanced computational techniques in chemistry. The future of reaction dynamics could very well depend on embracing these complex pathways that define how chemical transformations occur in nature.