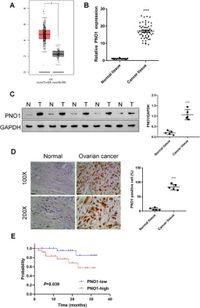
A study published on March 20, 2025, has made significant strides in understanding the role of PNO1, a promoter of oncogenesis, in ovarian cancer. Researchers affiliated with the Obstetrics & Gynecology Hospital of Fudan University found that high levels of PNO1 expression are not only prevalent in ovarian cancer tissues but are also linked to poorer survival rates among patients. This discovery sheds light on the complex mechanisms contributing to the aggressive nature of this malignancy, which ranks as one of the deadliest gynecological cancers.
According to analyses performed using The Cancer Genome Atlas (TCGA) database, PNO1 shows notably increased expression in ovarian cancer tissue compared to adjacent non-cancerous tissues. The study indicates that this upregulation of PNO1 enhances the tumorigenic potential through modulation of the AKT/Wnt/β-catenin signaling pathway. The findings suggest that targeting PNO1 may offer new therapeutic strategies in combating ovarian cancer, which often remains asymptomatic until its advanced stages, leading to a dire prognosis for many patients.
In various tests, silencing PNO1 in ovarian cancer cell lines was observed to substantially reduce their proliferation and invasion capabilities. This silencing induced apoptotic triggers across these cells, as the authors emphasized, "PNO1 silencing markedly reduced the proliferation and invasion capabilities of ovarian cancer cell lines, triggering their apoptosis." This reduction is further supported by observations in clinical specimens where patient survival rates dipped significantly with increased PNO1 expression. The high-expression group of patients showed a significantly poorer prognosis compared to those with the low-expression group of patients, reinforcing the significance of this gene as a potential target for treatment.
To dissect the mechanisms at play, the researchers employed advanced methodologies, including reverse transcription quantitative polymerase chain reaction (RT-qPCR) and Western blotting, revealing that knockdown of PNO1 not only dampened cellular proliferation but also decreased the expression levels of crucial proteins like phosphorylated AKT (p-AKT), GSK-3β, and active β-catenin. In this context, PNO1 seems to hijack the AKT signaling pathway, a known contributor to various cancerous presentations.
Furthermore, the study provides insight into how the AKT/Wnt/β-catenin pathway's activation can be inhibited, thereby curbing the oncogenic capabilities of ovarian cancer cells. The implementation of the AKT pathway inhibitor, SC79, demonstrated an ability to counteract the adverse effects observed with PNO1 knockdown, confirming that the oncogenic effects mediated by the PNO1-activated Wnt/β-catenin pathway can indeed be rectified by inhibiting AKT signaling.
In parallel, when PNO1 was overexpressed in specific ovarian cancer cell lines, marked increases in cell proliferation, migration, and invasion were documented. The study findings emphasized the importance of oncogenes in the survival and aggressiveness of tumor cells, showcasing that "PNO1 overexpression significantly enhanced the malignant characteristics of cells." The implications of these findings are multi-faceted; not only do they advance scientific knowledge regarding ovarian cancer biology, but they also hint at clinical applications where targeting PNO1 could yield benefits in a treatment framework.
The in vivo models, particularly using nude mice to test the effects of PNO1 knockdown, revealed that tumors in the experimental group were significantly smaller compared to their counterparts with normal PNO1 expression, affirming the pivotal role PNO1 plays in sustaining tumor growth. The expression levels of PNO1 and Ki-67 in the tumor tissues were significantly lower post-knockdown, demonstrating potential avenues for therapeutic exploration.
In conclusion, this comprehensive study elucidates the oncogenic capacity of PNO1 within the realm of ovarian cancer, suggesting that PNO1 promotes the progression of this cancer through activation of AKT/Wnt/β-catenin pathways. These findings not only reinforce the need for continual research into ovarian cancer's molecular underpinnings but offer a targeted approach to its treatment. As the authors noted, "PNO1 influences the malignant phenotype of ovarian cancer cells through the AKT/β-catenin pathway," which could pave the way for future therapeutic interventions to improve patient outcomes.