Translating the complex landscape of pain perception into actionable insights has remained a central quest for neuroscientists. In recent research published on March 20, 2025, a team of scientists at the University of Glasgow has identified and characterized a distinct population of spinal neurons that express somatostatin, implicating these neurons in the multifaceted dimensions of pain. This research not only sheds light on the intricacies of pain processing in the nervous system but also raises pertinent questions regarding effective interventions.
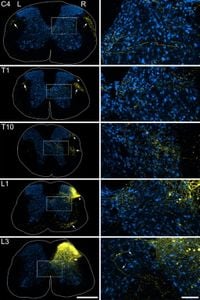
Known as the anterolateral system (ALS), the spinal projection neurons labeled as ALS4 play a crucial role in how pain is perceived and processed in our bodies. The study utilized a genetically modified mouse line, specifically the SstCre mice, allowing researchers to pinpoint and explore the specific characteristics and axonal projections of somatostatin-expressing neurons in detail. Notably, these neurons are predominantly situated in the lateral portion of lamina V within the spinal cord.
According to the study's findings, these ALS4 neurons primarily ascend along the ipsilateral side of the spinal cord, establishing a significant bundle within the ipsilateral ventral funiculus, particularly seen at upper lumbar levels. This contrarian pattern of ascending projections contrasts norms in spinal cord neuroscience, wherein many projection neurons predominantly cross over to the opposite side before ascending.
In their investigations, the researchers revealed that ALS4 neurons demonstrate extensive axonal projections, with fibers stretching into several brainstem nuclei, including the parabrachial internal lateral nucleus and the posterior triangular thalamic nuclei. Importantly, approximately 75% of ALS neurons in the lateral lamina V express somatostatin, highlighting a substantial population of these cells—around 120 somatostatin-positive neurons—on each side of the spine's L4 segment.
"Our findings indicate that this is a relatively large population, and based on projection targets, we conclude that they are likely to contribute to the affective-motivational dimension of pain," wrote the authors of the article. This statement encapsulates the role of these neurons, underlining how they may influence not just the sensation of pain but the emotional response associated with it as well.
The implications of these discoveries are profound. Understanding how these neurons contribute specifically to pain processing could lead to novel therapeutic approaches for managing chronic pain conditions. As the scientists mapped the projections of the somatostatin-expressing ALS4 neurons, they painted a clearer picture of complex pain circuitry that has remained obscure for years.
Typically, pain perception is transmitted through various neural pathways, and the spinal dorsal horn plays a pivotal role in processing somatosensory information. Through a network of interneurons and projection neurons, the dorsal horn processes signals from the body's tissues before sending this information onward to the brain for higher processing. Somatostatin-expressing neurons appear to serve as critical mediators in this process, potentially influencing how pain is interpreted, felt, and reacted to.
The methodological framework of this research involved sophisticated genetic targeting and anterograde tracing, providing nuanced insights into how specific populations of spinal neurons interact within established pain pathways. These methods not only confirmed the significant presence of somatostatin within the ALS pathway but also indicated the intricate connecting fibers that intersect various regions of the brain involved in pain processing.
Furthermore, as the scientific community evaluates the study's contributions, many are likely to further explore the therapeutic potential of targeting somatostatin-expressing neurons within clinical settings. In choosing to trace out these neurons, researchers have opened pathways for targeted pain relief strategies that could bypass some of the systemic complications associated with traditional pain management approaches. In fact, accurate manipulation of these pathways could lead to significant advancements in how we treat and understand pain across diverse medical fields.
As neuroscientists continue to unravel the complexities of the nervous system, the research surrounding somatostatin-expressing ALS4 neurons stands as a promising step toward more personalized pain treatment options, highlighting their potential substantial impact across various neurobiological frameworks. Researcher's insights into the relation between somatostatin-expressing neurons and the affective dimensions of pain signify an exciting frontier in our understanding of pain as a construct shaped as much by biology as it is by experience.
In summary, this groundbreaking research serves as a clarion call for a deeper understanding of how specific neural populations interact to shape our experience of pain. With methodologies that allow for pinpoint accuracy in identifying and categorizing neuron types, the horizon of pain neuroscience is expanding—heralding a future where targeted therapies become a reality in managing what is arguably one of the most complex facets of human health.