A novel organic electrode material has been developed for aluminum-ion batteries (AIBs) that significantly enhances their energy capacity through multielectron transfer. Researchers have introduced a tetraalkynylporphyrin macrocycle positive electrode, which has been shown to achieve multielectron transfer, leading to battery capacities reaching 347 mAh g−1, significantly higher than the traditional graphite electrodes which typically range around 60-120 mAh g−1.
Rechargeable aluminum-ion batteries are being hailed as a revolutionary advancement in electrochemical energy storage devices due to their high volumetric capacity, but they have faced challenges with batteries' actual capacity and cycling lives due to inadequate electrode materials. While much effort has gone into developing various inorganic materials like carbon structures and transition metal compounds, electrochemical performances have still been deemed unsatisfactory.
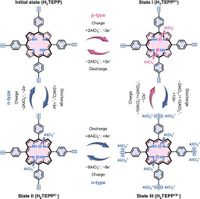
Emerging as a solution, organic electrode materials have demonstrated diverse redox activities and flexible molecular designs. Specifically, the bipolar property of the newly synthesized tetraalkynylporphyrin allows an impressive 12-electron transfer charge storage mechanism for AIBs. This innovative design includes two p-type amine motifs and two n-type imine motifs facilitating the alternation of binding and releasing AlCl4− anions and AlCl2+ cations during charging and discharging respectively.
The research team, led by studies published in March 2025, synthesized the complex and characterized its electrochemical properties, revealing that the tetraalkynylporphyrin electrode could bond and release aluminum ions effectively, achieving a high capacity of 347 mAh g−1 and a specific energy of 312 Wh kg−1. These metrics are substantial gains over previous benchmarks and highlight the efficiency of the multielectron transfer mechanism.
However, traditional inorganic electrodes, while having reasonably good cycling life and specific energy, still struggled with issues such as capacity decay and dissolution of active materials in ionic liquid electrolytes. The new organic electrode demonstrates not only improved capacity but also outstanding cyclability, retaining 87% of its capacity after 6500 cycles.
This research heralds a promising strategy for designing multielectron-transfer bipolar-redox organic positive electrode materials to realize high-energy aluminum-ion batteries. The unique properties of tetraalkynylporphyrin enable effective coordination with Al-complex ions, significantly addressing the performance limitations of existing technologies.
In summary, the bipolar nature and multielectron transfer capabilities of the newly developed tetraalkynylporphyrin offer a vital improvement in the quest for high-energy, sustainable battery technologies. Researchers are optimistic that this advancement will pave the way for future developments in energy storage systems, particularly in the realm of aluminum-ion batteries.