In a groundbreaking advance for genetic research, scientists have successfully developed a non-viral method for generating transgenic cynomolgus monkeys, a leap forward that could enhance the study of human diseases and development.
This innovative technique employs the piggyBac transposon system, allowing researchers to introduce genetic material into monkeys without the ethical complications associated with viral methods. Prior approaches, particularly those using lentiviral vectors, have faced significant hurdles, including the need for specialized equipment, challenges in embryo screening, and issues with genetic mosaicism—a situation where some cells carry the transgene while others do not.
Cynomolgus monkeys are an essential model for understanding human biology due to their close genetic relationship with humans. Traditional methods for creating genetically modified monkeys often involve labor-intensive and inefficient techniques, making the new non-viral piggyBac method a promising alternative.
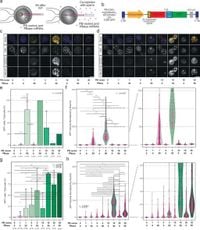
Research led by a team of international scientists aimed to optimize the piggyBac system after preliminary success in mice. Through a rigorous experimental design involving co-injecting piggyBac components with sperm into metaphase II oocytes, they determined the optimal conditions that would allow successful transgene expression throughout the body of the monkeys.
The researchers began their experiments with extensive protocols in mice, establishing that concentrations of 10 ng/μL of the piggyBac vector resulted in efficient transgene integration. This concentration proved optimal in ensuring embryo viability and transgene expression. Following initial success, the team proceeded to apply this method to cynomolgus monkeys, achieving similarly impressive results.
Among the 34 blastocysts created, 32 tested positive for the introduced transgene, suggesting high efficiency in the transgenesis process. Notably, a careful approach was taken to identify male embryos before transfer, which was crucial for planning future breeding studies.
Following the cohort of embryos through to live births, the team reported the birth of three monkeys that displayed the desired genetic modifications. Unfortunately, one of these monkeys died shortly after birth, highlighting the unpredictability of outcomes in genetic modeling; however, necropsy confirmed robust transgene expression across all analyzed tissues.
The methodology’s substantial advancement lies in its simplicity and ethical considerations. Unlike viral methods that present biosafety concerns, the piggyBac system eliminates the need for viral vectors, streamlining the process for generating genetically modified animals. This breakthrough aligns with a growing demand for ethically sound research practices in the biomedical field.
This study marks a significant milestone in the field of genetic engineering, with implications that extend beyond primate studies to potentially enhance the production of transgenic animals in agriculture, biotechnology, and medicine. By providing a reliable method to create transgenic monkeys, researchers hope to pave the way for more insightful studies into human diseases that mirror real-world physiology.
The implications of this research are vast—scientific conversations continue about the role of such models in not only understanding genetic diseases but also in advancing the efficacy of drug development and safety testing.
Ultimately, the promise of this new technique rests in its potential to significantly bolster our understanding of human development and inform strategies to mitigate diseases that affect millions worldwide.