In the race against Alzheimer's disease (AD), a recent study published on March 21, 2025, sheds light on promising advancements in diagnosing this debilitating condition. The study introduces a cytometric bead array (CBA) method for measuring crucial plasma biomarkers associated with AD, specifically amyloid-β (Aβ) peptides and phosphorylated tau (P-tau). As the prevalence of Alzheimer’s is expected to reach nearly one million new cases annually by 2050, the need for effective, non-invasive diagnostic tools is more pressing than ever.
Alzheimer’s disease is characterized by a decline in cognitive function and daily activities, impacting millions of elderly individuals worldwide. The condition accounts for approximately 60 to 70 percent of dementia cases, and early diagnosis remains vital for successful intervention strategies. Traditional diagnostic procedures, particularly positron emission tomography (PET) scans and cerebrospinal fluid (CSF) analysis, are often limited by their invasiveness or resource demands, prompting researchers to explore more accessible methods.
The study, conducted at Tianjin Medical University General Hospital, evaluated the diagnostic accuracy of the CBA method, examining plasma samples from fifty patients with cognitive impairment (CI) and twenty-two cognitively unimpaired (CU) controls. The CI patients were categorized into two groups—Aβ-positive (Aβ + CI) and Aβ-negative (Aβ - CI)—based on their amyloid PET scan results. This classification is critical, as Aβ accumulation in the brain is a hallmark of Alzheimer’s disease.
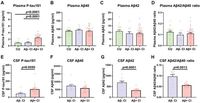
Results indicated that plasma P-tau181 levels were markedly higher in the Aβ + CI group compared to both the Aβ - CI group and the CU controls, showcasing its potential as a distinguishing biomarker. Specifically, the plasma P-tau181 level exhibited an area under the receiver operating characteristic curve (AUC) of 0.9043 with a sensitivity of 0.842 and specificity of 0.909, indicating excellent diagnostic performance for identifying Aβ + CI patients. As the study highlights, "Plasma P-tau181 levels were greatest in the Aβ + CI group and showed excellent performance in differentiating Aβ + CI patients from CU controls."
In total, thirty-eight CI patients were classified as Aβ + CI, with higher levels of P-tau181 and lower levels of Aβ42 than observed in the Aβ - CI group. These findings were corroborated by analyzing CSF samples from twenty-eight CI patients, where similar differences in P-tau181 levels and Aβ42/Aβ40 ratios were noted. The study’s authors point out, "In our study, CSF biomarkers, including P-tau181, Aβ42 and the Aβ42/Aβ40 ratio, assayed by the CBA method were significantly different between Aβ + CI patients and Aβ- CI patients." This high degree of correlation between plasma and CSF levels of tau proteins suggests that CBA could offer a reliable method for assessing AD-related pathology, with substantial implications for both diagnosis and patient monitoring in clinical settings.
The correlation between plasma P-tau181 levels and cognitive function was also investigated, revealing a significant negative association with scores on the Mini-Mental State Examination (MMSE). This indicates that as P-tau181 levels increase, cognitive performance tends to decline. The authors noted the plasma P-tau181 level was correlated with the MMSE score, further reinforcing its potential role as a peripheral biomarker reflective of clinical progression in Alzheimer’s disease.
Compared to traditional methods like PET scans, the CBA method offers several advantages, including cost-effectiveness and ease of obtaining blood samples, which may improve accessibility in a broader range of clinical environments. The researchers assert that "The measurement of plasma P-tau181 with the CBA method... has potential value in the diagnosis, differentiation and monitoring of AD." Such methodologies could pave the way for routine screening and early intervention in at-risk populations, potentially altering the trajectory of Alzheimer’s disease management.
As the Alzheimer's research community continues to seek innovative solutions to the challenges posed by AD, the findings from this study propel blood-based biomarker research forward. The promising results demonstrate the CBA method's capability to accurately detect critical biomarkers in Alzheimer’s disease, thereby increasing the potential for earlier diagnosis and better outcomes for patients. Nevertheless, further research with larger sample sizes and diverse populations is needed to validate these findings and refine the application of CBA in clinical practice.