In a groundbreaking study published in Nature Communications, researchers have unveiled a novel method for creating high-density single-atom catalysts, a significant advancement in efficient oxygen evolution reactions (OER). This innovative approach centers around the integration of iridium atoms within ultrathin cobalt-cerium oxyhydroxide (CoCeOOH) nanosheets, marking a leap in catalyst design that could reshape renewable energy technologies.
The research, conducted by a team from the City University of Hong Kong, highlights the effective use of metastable iridium species during the electrochemical synthesis process. The resulting cobalt-cerium iridium single-atom catalyst (CoCe-O-IrSA) demonstrates unprecedented efficiencies, achieving an overpotential of just 187 mV at a current density of 100 mA cm−2, while maintaining stable performance for over 1000 hours at higher current densities. This durability is crucial in practical applications, especially in promoting efficient water splitting processes essential for sustainable energy production.
As the pressure mounts on the scientific community to develop efficient catalysts that address the challenges of sluggish kinetics in OER, the team led by Q.Q. and J.C.H. has provided an innovative solution. By fostering a structural evolution that maximizes metal-substrate interactions through electrochemical methods, the researchers successfully achieved a robust catalytic material capable of high-density functionality in ambient conditions.
The necessity for high-performance OER catalysts stems from their central role in various renewable energy applications, including water electrolysis, carbon dioxide reduction, and metal-air batteries, all of which require catalysts to operate efficiently without excessive energy losses. Previous attempts to create high-density single-atom catalysts faced significant hurdles; individual metal atoms often agglomerate into larger clusters or nanoparticles due to weak metal-substrate interactions. This new methodology, however, effectively mitigates these challenges.
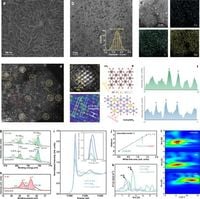
The preparation of the CoCe-O-IrSA involved an electrochemically initiated synthesis that allowed iridium to maintain a stable single-site configuration, ensuring its active participation in the OER process. By utilizing symmetry-breaking CoCe(OH)2 substrates, the iridium species were embedded efficiently into a high-density arrangement, exhibiting exceptional catalytic properties. In their findings, the authors noted that "the reconstructed thermodynamically stable iridium single atoms act as highly active sites by regulating charge redistribution with strongly p-d-f orbital couplings," underscoring the advanced electronic structure that enhances catalytic performance.
During testing, the CoCe-O-IrSA exhibited remarkable resilience as an anode in an anion-exchange-membrane water electrolysis system for seawater splitting, sustaining high current densities of 500 mA cm−2 for 150 hours. Such longevity suggests that this new catalyst could be pivotal in real-world applications, particularly in energy systems utilizing seawater—a resource that remains largely untapped in current electrolysis technologies.
This research opens new avenues for engineering metastable phases to achieve stable single-atom systems designed for effective energy-conversion applications. The ability to maintain high-density structures without compromising stability lays a foundation for further exploration and development in catalytic technologies focused on renewable energy.
In conclusion, the advances introduced by the CoCe-O-IrSA highlight the ongoing need for innovative catalyst designs capable of tackling significant energy challenges. The successful integration of metastable phases into high-performance catalysts underscores the critical intersection of materials science and electrochemistry, paving the way for a future where efficient energy conversion is a reality.