In the race to combat air pollution and climate change, researchers have developed a groundbreaking method for capturing acid gases using a newly designed material. The material, a single-crystalline-like porous carbon known as Zn-N3@SC-PC, demonstrates an unprecedented ability to adsorb acid gases like CO2, COS, and H2S with a remarkable stoichiometry that exceeds the traditional 1:1 ratio, making it a promising frontier in environmental science.
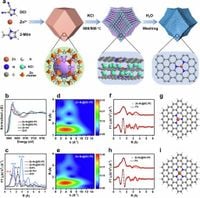
The study describes how this innovative material is synthesized through a precise carbonization process of ZIF-8-C ≡ N in the presence of KCl, yielding a structure that enables multi-molar adsorption capabilities. Specifically, the Zn-N3@SC-PC can capture up to 6 SO2 molecules per concentration unit, significantly enhancing gas separation efficiency compared to conventional porous adsorbents.
Reporting in Nature Communications, a team of scientists has revealed the impressive characteristics of Zn-N3@SC-PC. Notably, this structure has a high surface area, showing an ability to maintain 21.2 mmol/g SO2 adsorption under controlled conditions. This provides both a solution to environmental challenges posed by acid gas emissions and a sustainable option for industries that rely on natural gas and other processes that emit harmful gases.
“Historically, attempts to capture acid gases relied heavily on amine scrubbing processes that often presented low efficiency and posed risks such as corrosion and complex regeneration,” stated the authors of the article. “Our advancement offers a robust alternative that not only reduces these concerns but also optimizes adsorption properties.”
The research also highlights how the team succeeded in addressing existing challenges associated with capturing acid gases efficiently. Achieving an adsorption capacity greater than 1:1 interaction has long been a barrier. The innovative design of Zn-N3@SC-PC, with its unique protruded structure, enables multiple interaction sites through vacant coordination orbitals, effectively breaking the conventional stoichiometric limits of acid gas interaction.
To construct the Zn-N3@SC-PC, the researchers employed various characterization techniques, including X-ray diffraction (XRD) and Fourier Transform Infrared Spectroscopy (FT-IR), together with advanced spectroscopic methods like Extended X-ray Absorption Fine Structure (EXAFS), which confirmed the material's atomic structure and functional capacity. Results marked a success, as Zn-N3@SC-PC demonstrated a unique coordination environment that enables it to outperform previous adsorbent materials.
Moreover, Zn-N3@SC-PC exhibited stability during 50 adsorption-desorption cycles, highlighting its applicability in real-world scenarios where the continuous capture and release of gases are essential. This trait is “crucial for practical applications in industrial settings,” as emphasized in the research.
Notably, the newly designed material is not limited to SO2 capture alone; it also achieves significant adsorption for other acid gases such as CO2 and H2S, making it a potential contender for broader environmental application areas, including greenhouse gas management and industrial exhaust purification.
“This novel approach decouples the standard constraints of adsorbent materials, fostering opportunities for enhanced carbon capture technologies that can significantly mitigate the adverse effects of anthropogenic emissions,” the authors of the article added.
As climate change and environmental degradation continue to challenge global health and ecosystems, innovations like Zn-N3@SC-PC represent an important stride toward creating sustainable solutions for air quality management and carbon neutrality. By breaking the traditional limitations of acid gas capture, this research may pave the way for future studies aiming to hone and expand upon these findings, allowing for exploration into even more effective materials.
In summary, the Zn-N3@SC-PC presents a substantial advancement in gas capture technology, showcasing a method that could very well change the landscape of acid gas separation and carbon management. This research not only sheds light on effective mitigation strategies for pressing environmental issues but also inspires future innovations by setting a new benchmark for performance in gas adsorption.