The ability to visualize chemical changes at the molecular level has long been a goal for scientists, allowing for a deeper understanding of chemical reactions and molecular dynamics. Researchers at various institutions have made a significant advancement in this field by developing a method that enables simultaneous imaging of nuclear and electronic dynamics in small molecules, using ultrafast laser techniques.
This study focuses on the dissociative photoionization of two important diatomic models, hydrogen (2) and nitrous oxide (N2O). By employing time-resolved photoion-photoelectron coincidence spectroscopy, the researchers aim to capture the fleeting moments during which chemical bonds break and molecular structures transform.
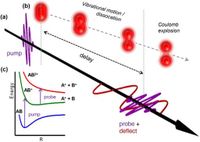
To probe these ultrafast phenomena, the team harnessed femtosecond and attosecond laser technology, allowing them to resolve changes in molecular dynamics on extremely short timescales. "Our work shows opportunities and challenges on the track towards capturing simple gas-phase chemical dynamics in complete molecular movies," wrote the authors. The researchers combined Coulomb Explosion Imaging (CEI) with pump-probe techniques to visualize the nuclear motion while also monitoring the transient electronic structure of the molecules involved.
Throughout the experiment, the pump pulse ionizes the hydrogen molecule, creating H2+, with the probe pulse then monitoring the nuclear and electronic motions of this resulting ion. The team observed that delay-dependent kinetic energy release distributions revealed two primary processes occurring after the ionization: bound vibrational motion, observed at high kinetic energies (KER > 4 eV), and dissociation, characterized by lower energies and a narrow distribution that decays with increasing delay as the bond breaks.
Moreover, the variations in electronic structure associated with nuclear dynamics were explored by directly imaging outgoing electron wave packets. Using calculations for H2+, the researchers systematically analyzed the relationship between internuclear distances and the resulting photoelectron momentum distributions (PMDs). This analysis demonstrated significant changes in electronic distributions as the molecular bond stretched and eventually broke.
The experiment's findings have broader implications, particularly when considering the dynamics of polyatomic molecules. In the case of N2O, the more complex diatomic bond structures allow for several dissociation channels, notably along the N-N and N-O bonds. There, the resulting fragments can lead to the formation of various ionic products, influencing the overall dissociation dynamics observed in the study. By isolating specific dissociation events through careful experimental design, the researchers hope to deepen the understanding of these complex processes.
The experimental setup for H2 and N2O utilized a femtosecond Ti:Sa laser, enabling highly energetic and precise interactions with the molecular samples. It is noted that the peak intensities of the pump and probe pulses were approximately 3 × 10^14 W/cm², facilitating efficient ionization and subsequent imaging of the resultant molecular fragments.
Through rigorous computational modeling alongside experimental observation, the researchers were able to characterize the electronic states involved in the dissociation processes and identify the significant role that electron localization plays during these dynamic events. The results underscore the stark contrast between the behavior of simpler diatomic hydrogen and the more intricate systems represented by nitrous oxide.
Importantly, the investigations revealed that the photoelectron images retained clear indicators of the molecular orbital character, hinting at how electron density redistributes during fragmentation processes. This highlights one of the method's potential breakthroughs: a refined approach to building a complete picture of a molecule's transformation during chemical reactions.
Furthermore, while the study primarily addressed ultrafast processes in diatomic molecules, the implications extend towards potential applications in visualizing more complex molecular systems and understanding how different conditions affect chemical dynamics.
In conclusion, the researchers continue to refine their experimental techniques and theoretical models, hoping to increase the time resolution and extent of their observations in capturing molecular dynamics. The groundbreaking nature of this work lies in its promise for developing complete molecular movies that capture the continuous flow of both nuclear and electronic changes during chemical reactions, thus paving the way for future discoveries in molecular chemistry.